Abstract
Background
Complete blood count (CBC) and white blood cell (WBC) differential are essential tests for various diseases. Related to this, the Mindray BC-6200 automated hematology analyzer (BC-6200, Mindray Bio-Medical Electronics Co., Ltd, China) was recently launched in clinical laboratories. This study aimed to evaluate the analytical and flagging performance of BC-6200.
Methods
Using 688 whole blood samples, the precision and carryover of 12 CBC parameters were evaluated with BC-6200 according to the Clinical and Laboratory Standards Institute (CLSI) guidelines EP15-A3 and H26-A2, respectively. 11 CBC parameters of BC-6200 were compared with Sysmex XE-2100 (XE-2100, Sysmex Corporation, Japan) according to the CLSI guideline EP09c. To evaluate the flagging performance for blasts, immature granulocytes (IG), atypical lymphocytes (AL), and nucleated red blood cells (NRBC) of BC-6200, sensitivity, specificity, and efficiency to manual counts were estimated according to the CLSI guideline H20-A2.
Results
Precisions of WBC, red blood cells (RBC), hemoglobin (Hb), hematocrit (Hct), and platelets (PLT) were acceptable. Carryover was less than 1% in WBC, RBC, Hb, Hct, and PLT. In WBC differentials, BC-6200 and XE-2100 showed very high correlations, except for basophils. Flagging performances of BC-6200 showed excellent results in efficiency; 91.4% for blasts, 79.4% for IG, 75.5% for AL, and 98.6% for NRBC.
초록
배경
전체혈구계산과 백혈구 감별계산은 다양한 질병의 진단에 있어서 필수적인 검사이다. 이에 따라 본 연구에서는 최근 임상 검사실에 도입된 자동혈구분석기인 Mindray BC-6200 (BC-6200, Mindray Bio-Medical Electronics Co., Ltd, Shenzen, China)의 분석 수행능력 및 flag 지정능력을 평가하고자 한다.
방법
총 688개의 전혈 검체를 이용하여 Clinical and Laboratory Standards Institute 지침에 따라 BC-6200의 검사 성능을 평가하였다. 12개의 전혈구계산 항목에 대해 BC-6200의 정밀도 및 잔효를 평가하였으며, 11개의 전혈구계산 항목에 대해 기존 보유 자동혈구분석기인 Sysmex XE-2100 (XE-2100, Sysmex Corporation, Kobe, Japan)과의 상관성을 평가하였다. 또한 BC-6200의 모세포, 미성숙 과립구, 비정형 림프구, 유핵적혈구 flag 지정능력을 평가하기 위하여 해당 항목들에 대하여 현미경 수기법과 비교하여 민감도, 특이도, 효율성을 평가하였다.
Abnormal complete blood count (CBC), white blood cell (WBC) differentials, or the presence of immature blood cells are known to be associated with hematologic disorders and a wide variety of cardiovascular diseases and even metabolic disorders [1, 2]. For these matters, laboratories need automated hematology analyzers (HA) that can process a flurry of data rapidly, let alone with high precision. Such HA should be able to identify abnormal results, report them, and induce manual confirmation if required.
Since the 1970s, when 3-part WBC differentials were first available, recent technological advancements have led HA increasingly complex, and various analyzers were developed using new methods such as fluorescent dye or flow cytometry in conjunction with traditional methods like electrical impedance and laser light scattering [3, 4]. As a result, HA progressed to the stage where they are capable of counting 5-part WBC differential, identifying abnormal cells such as blasts, immature granulocytes (IG), atypical lymphocytes (AL), or nucleated red blood cells (NRBC). These abnormal cells are mostly seen in hematology-related patients, as well as due to the cellular interferences or technical problems of the system [5]. Nowadays, automated HA are one of the most essential parts of clinical laboratories, and manufacturers worldwide are working to improve the instruments.
The recently developed Mindray BC-6200 (BC-6200, Mindray Bio-Medical Electronics Co., Ltd, Shenzhen, China) is an automated HA that can measure not only peripheral blood samples but also numerous kinds of body fluid samples through various methods and multiple platforms. This study was conducted to evaluate the basic performances and analytical abilities of BC-6200 for major CBC parameters, including a comparison with the currently used HA, Sysmex XE-2100 (XE-2100, Sysmex Corporation, Kobe, Japan), and the assessment of abnormal cell flagging performance. As far as we know, only one other evaluation study of BC-6200 was done previously [6]. However, the study lacked an evaluation for the flagging performance of NRBC. The presence of NRBC in the blood is associated with extramedullary hematopoiesis and often suggests bone marrow abnormalities [7]. Detecting NRBC also has high prognostic power regarding mortality in critically ill patients [8]. These reflect how important NRBC detection is in clinical laboratories; therefore, our study included NRBC as part of the assessment for flagging performance.
From May to September 2018, 688 blood samples were collected to conduct this study. In particular, 596 blood samples were collected consecutively from outpatients and inpatients of Konkuk University Chungju Hospital (KUCH). For the assessment of flagging performance, an additional 92 blood samples from patients at Konkuk University Medical Center (KUMC) were included. These additional samples were from patients diagnosed with hematologic diseases, such as acute myeloid leukemia, myelodysplastic syndrome, or lymphoma, with most of them undergoing chemotherapy. Samples from KUMC were transported to KUCH within 3 hours in a refrigerated state. Evaluated CBC parameters in this study were WBC, red blood cells (RBC), hemoglobin (Hb), hematocrit (Hct), red cell distribution width-coefficient of variation (RDW-CV), platelets (PLT), reticulocytes, neutrophils, lymphocytes, monocytes, eosinophils, and basophils (Table 1). The flow of the total study population is summarized as a diagram in Fig. 1. There was no restrictive sample collection regarding age or sex, and the samples were not collected exclusively for this evaluation project. EDTA-K2 and EDTA-K3 anticoagulant tubes were each used to collect venous blood samples in KUCH and KUMC, respectively. Samples that were coagulated or became hemolytic were excluded in the evaluation process. Samples from KUCH were run in BC-6200 and/or XE-2100 analyzers within 4 hours after collection. Samples transported from KUMC were run also within 4 hours. This study was exempted from deliberation by the Institutional Review Board of KUCH (KUCH 2018-05-022).
BC-6200 is an automatic HA capable of quantifying CBC, WBC 5-differentiation (5-diff), reticulocytes, and NRBC. BC-6200 is capable of performing 110 tests per hour, which is enough to be used in mid-level and secondary hospitals. It uses the new innovative “SF Cube” technology, a method based on the front and side scattering (S) and fluorescence (F) of the DNA and/or RNA in the target cell [9-11]. As a result, it ensures more accurate results by creating a greater distance between each cell group in building 3D scattering diagrams. Through the DIFF channel, BC-6200 provides information on major CBC parameters and that of blasts, AL, IG, immature reticulocytes, and immature platelets. With the WNB channel, BC-6200 provides results of NRBC or basophils. Through the RET channel, it measures reticulocytes, immature reticulocyte fraction, or immature platelet fraction by using the SF cell technology. Reticulocytes were included as a part of the parameters tested for within-run precision, as the RET channel was an added feature of BC-6200 to the previous version, BC-6000. Note that compared with the advanced version, BC-6800Plus, BC-6200 is suitable in secondary hospitals due to its more compact size and lesser sample requirements.
The reference analyzer XE-2100 also measures samples in different methods and channels. Fluorescence flow cytometry method is used in the DIFF and NRBC channels. WBC 5-diff and IG counts are derived from the DIFF channel, and a specific NRBC channel is used to measure NRBC. Through the reticulocyte channel, the direct current sheath flow method is used to measure RBC, reticulocytes, and PLT [12].
Within-run repeatability was evaluated with three samples according to the Clinical and Laboratory Standards Institute (CLSI) guideline EP15-A3 [13]. The three samples were each tested 10 consecutive times within a few minutes. The mean, standard deviation (SD), and coefficient of variation (CV) of the 12 CBC parameters were calculated.
Carryover was evaluated using high and low levels of fresh whole blood provided by the manufacturer. According to the CLSI guideline H26-A2 [14], high-level samples were measured three consecutive times followed by immediate measurements of three consecutive low-level samples. The percentage carryover was calculated using the formula below:
Carryover%=(L1-L3)/(H3-L3)×100
A total of 305 samples were analyzed in duplicates on both BC-6200 and XE-2100 according to the CLSI guideline EP09c [15]. However, 35 samples had partial missing data, and 270 samples were evaluated as a result. The correlation coefficient, slope, intercept, and P-value were each calculated for WBC, RBC, Hb, Hct, RDW-CV, PLT, and WBC differential parameters. Bland-Altman plots for each parameter were also drawn for visual comparison.
Among 380 samples gathered for assessing flagging performances, 21 samples were excluded due to improper operation of the instrument. For the remaining 359 samples, BC-6200 flagged blasts, IG, NRBC, or AL. According to the CLSI guideline H20-A2 [16], samples were analyzed in duplicate, and the flagging was considered positive only when both samples had the same flags. For manual microscopy, two separate blood smears were each examined by two trained laboratory technicians. A third copy of the smear was reserved in case of any arbitration purposes. If blasts ≥1% were found in the manual microscopy of both technicians, the sample was considered blast-positive. Same was true for IG (myelocyte ≥1%, metamyelocyte >2%), NRBC (≥1%), and AL (>5%). If there were any disagreements between the two manual results, the third smear was reviewed by the arbitrator. The sensitivity, specificity, and efficiency were calculated for each flag.
CBC parameters of the total study population were presented in the form of median and interquartile ranges. Precision results for the determined parameters were expressed in mean, SD, and CV. Reviews by Vis et al. [17] were used in reference to verify whe-ther the measured CVs reached the current state-of-the-art criteria. Spearman’s correlation coefficient, slope, and intercept were accounted for the translation of the comparison study. Correlation coefficient values were interpreted using the rule of thumb provided by Mukaka [18] and were considered as follows: ≥0.90 as very high correlation; 0.70–0.90 as high correlation; 0.50–0.70 as moderate correlation; 0.30–0.50 as low correlation; and ≤0.30 as negligible correlation. Bland-Altman analysis depicting mean differences and 95% limits of agreements was also used to aid in comparison. The sensitivity, specificity, and overall efficiency was calculated from the number of true positives (TP), false positives (FP), true negatives (TN), and false negatives (FN) as follows: Sensitivity (%)=TP/(TP+FN)×100; specificity (%)=TN/(TN+FP)×100; effici-ency=(TP+TN)/(TP+FP+TN+FN)×100. P values of <0.05 were considered statistically significant. MedCalc Statistical Software (version 20.015, MedCalc Software Ltd., Ostend, Belgium) was the tool for achieving data analysis in this study.
The within-run precision results for WBC, RBC, Hb, Hct, RDW-CV, PLT, reticulocytes, and WBC 5-diff are shown in Table 2. Two samples had parameters within normal levels (samples 1 and 2), and one sample had cytopenic features (sample 3). For each sample, the CVs of WBC, RBC, Hb, Hct, RDW-CV, PLT, and reticulocytes were all acceptable. In WBC 5-diff, the CVs of the monocyte count were all acceptable, but others had different results. In sample 1, the lymphocyte, eosinophil, and basophil counts were out of range. In sample 2, the lymphocyte count was out of range. In sample 3, the neutrophil, eosinophil, and basophil counts were out of range.
The percentage carryovers for the WBC, RBC, Hb, Hct, and PLT were as follows: -0.04%, 0.41%, 0.00%, 0.00%, and 0.28%, respectively. These results were all within the manufacturer’s acceptance criteria of ≤1% (data not shown).
Of the 270 whole blood samples analyzed, a comparison of results between the two HA is shown in Table 3. The parameters showed correlation coefficients above 0.90, except for that of basophils, which was revealed as 0.66. Fig. 2 shows the comparison results between the two analyzers in Bland-Altman plots.
The flagging performance results of BC-6200 compared to manual microscopy with 359 samples are shown in Table 4. A total of 78 samples were confirmed to have at least 1% blast count by manual slide review, and the BC-6200 showed blast flagging in 69 samples among them (sensitivity 88.5%). With 281 samples that were negative for blasts in manual count slides, BC-6200 showed blast flagging in 22 samples (specificity 92.2%). Microscopic evaluation revealed the presence of IG (myelocyte ≥1%, metamyelocyte >2%) in 34 samples, and BC-6200 correctly flagged them in 30 samples (sensitivity 88.2%, specificity 78.5%). The sensitivities of BC-6200 compared with a manual review for AL and NRBCs were 75.0% and 80.0%, respectively. The specificities for AL and NRBCs were 75.5% and 98.6%, respectively.
This study was conducted to evaluate the performance of BC-6200 in measuring CBC parameters and flagging abnormal results. In particular, CBC parameters, including WBC 5-diff, were compared to those of XE-2100, and the flagging performance was evaluated compared to the manual slide review. HA requires high sensitivities for detecting abnormal cells, as they are primarily used for screening. Therefore, the analytical performance of instruments should be evaluated before any use in laboratories. As the managers of clinical laboratories, we are responsible for seeking improvements and replacing any existing methods or hardware. Here we report an evaluation following our recent launch of BC-6200 in KUCH.
Carryover is considered a basic performance characteristic in evaluating HA, as an increased carryover would cause medical concerns such as falsely elevated results in subsequent cytopenic samples [14]. Other studies regarding BC-6200 [6] and XE-2100 [19] have reported percentage carryover results for major CBC parameters, all less than 1% as well. We can also say that our almost complete absence of carryover gives credibility to the following results.
The precision results for parameters other than the 5-diff were perfect for reaching the current state-of-the-art criteria. The CVs were satisfactory for neutrophil, lymphocyte, and monocyte counts. However, eosinophil and basophil counts had outlying results. That said, eosinophil and basophil counts were very low in these samples. Other authors admit that cell counting in samples with extremely low cell concentration often yields high imprecision [20]. Accurate basophil count is very hard to achieve by HA, as reported in other previous studies and reviews [21, 22]. Additionally, Kim et al. [23], with an evaluation study, including XE-2100, stated that a low concentration of basophils in the blood samples may explain the poor relationship between the methods.
BC-6200 demonstrated comparable CBC parameter results with those of XE-2100 (Table 3). Each of the parameters, except for the basophil count had very high correlations. Basophil count was considered moderately correlated between the two HA, showing a correlation coefficient of 0.66 [17]. Mean differences and 95% limits of agreement of the parameters showed no unusual trend, as depicted in the Bland-Altman plots (Fig. 2).
Basophil results in terms of correlation evaluations were not satisfactory as in the precision evaluation. This means that the manual slide reviews are still crucial in confirming the presence of basophilia in peripheral blood. Note that among the samples analyzed for correlation, the only basophilic sample was 0.28×109/L measured by XE-2100, but the corresponding BC-6200 data were 0.05×109/L. In that sense, future evaluation studies for more basophilic samples comparing BC-6200 with other HA or even manual counts would be interesting.
In the flagging performance results (Table 4), specificities and efficiencies of blasts, IG, AL, and NRBCs met the current state-of-the-art criteria of >70% and >75%, respectively, but sensitivities were slightly lower (criterion being >90% [14]). However, even though BC-6200 failed to flag blasts in 9 samples, it flagged them as IG instead. In this respect, BC-6200 did not miss any abnormal cells, making up for the slightly lower sensitivity values than the state-of-the-art criterion. Similarly, in four samples that were false negative for IG, three samples had neutrophils >80%, and one sample had a WBC count of 20.07×109/L. The sensitivity of AL is hard to consider as there were only four samples detected by manual microscopy. In the two samples that BC-6200 failed to identify NRBCs, both samples had IG and AL flags, meaning that those samples were still regarded as abnormal ones.
Although our study reached a satisfying goal, there were some limitations. First, we were unable to evaluate the comparison of reticulocytes between BC-6200 and XE-2100 due to the absence of corresponding data from XE-2100. As mentioned above, RET channel is the only addition from BC-6000 to BC-6200. Given that a previous study by Shen et al. [24] used the research-user-only version of BC-6000 for evaluation, including reticulocytes, a comparison study on this official product may have been useful. Second, our study evaluated the flagging performance of BC-6200 by comparing it with manual microscopy but did not have data on the comparison of XE-2100 with manual microscopy. Especially with parameters that showed a low correlation coefficient, such as the basophil count, a confirmed manual count would have made the results more confident. In the previously mentioned study by Kulik et al. [6], flagging performances of BC-6200 were compared with another HA and manual microscopy. XE-2100 has been used for several years in our laboratory with internal and external quality control protocols. Nonetheless, it would have been a more thorough evaluation as BC-6200 and XE-2100 have different methods in 5-diff counting.
In conclusion, the performance of BC-6200 based on the background, carryover, and precision results of CBC parameters were all excellent. CBC parameters were also well correlated with the standard instrument (i.e., XE-2100). The sensitivity, specificity, and efficiency of flagging were also acceptable. We conclude that BC-6200 is a competent HA to provide reliable and accurate diagnostic results and meets the needs of mid-volume testing in clinical laboratories.
REFERENCES
1. Libby P, Sidlow R, Lin AE, Gupta D, Jones LW, Moslehi J, et al. 2019; Clonal hematopoiesis: crossroads of aging, cardiovascular disease, and cancer: JACC review topic of the week. J Am Coll Cardiol. 74:567–77. DOI: 10.1016/j.jacc.2019.06.007. PMID: 31345432. PMCID: PMC6681657.
2. Orwoll ES, Orwoll RL. 1987; Hematologic abnormalities in patients with endocrine and metabolic disorders. Hematol Oncol Clin North Am. 1:261–79. DOI: 10.1016/S0889-8588(18)30675-0.

3. Briggs C, Culp N, Davis B, d'Onofrio G, Zini G, Machin SJ. 2014; ICSH guidelines for the evaluation of blood cell analysers including those used for differential leucocyte and reticulocyte counting. Int J Lab Hematol. 36:613–27. DOI: 10.1111/ijlh.12201. PMID: 24666725.

4. Sullivan E. 2006; Hematology analyzer: from workhorse to thoroughbred. LABMEDICINE. 37:273–8. DOI: 10.1309/TMQ6T4CBCG408141.

5. Park S, Huh J, Jeong TD. 2020; False-positive flag of WBC and change of mean platelet volume (MPV) caused by K3-EDTA on the DxH 900 hematology analyzer. Scand J Clin Lab Invest. 80:644–8. DOI: 10.1080/00365513.2020.1824298. PMID: 32975447.

6. Kulik K, Kwiecień I, Chełstowska B, Rutkowska E, Rzepecki P. 2021; Evaluation and comparison of the new Mindray BC-6200 hematology analyzer with ADVIA 2120i. Int J Lab Hematol. 43:395–402. DOI: 10.1111/ijlh.13418. PMID: 33270987.

7. Delsol G, Guiu-Godfrin B, Guiu M, Pris J, Corberand J, Fabre J. 1979; Leukoerythroblastosis and cancer frequency, prognosis, and physiopathologic significance. Cancer. 44:1009–13. DOI: 10.1002/1097-0142(197909)44:3<1009::AID-CNCR2820440331>3.0.CO;2-J.

8. Stachon A, Segbers E, Holland-Letz T, Kempf R, Hering S, Krieg M. 2007; Nucleated red blood cells in the blood of medical intensive care patients indicate increased mortality risk: a prospective cohort study. Crit Care. 11:R62. DOI: 10.1186/cc5932. PMID: 17550592. PMCID: PMC2206423.

9. Kim H, Hur M, Kim SW, Moon HW, Yun YM. 2020; Reference intervals for clinically reportable platelet parameters on the Mindray BC-6800Plus hematology analyzer. Clin Chem Lab Med. 58:e213–5. DOI: 10.1515/cclm-2020-0020. PMID: 32069230.

10. Deng J, Chen Y, Zhang S, Li L, Shi Q, Liu M, et al. 2020; Mindray SF-Cube technology: an effective way for correcting platelet count in individuals with EDTA dependent pseudothrombocytopenia. Clin Chim Acta. 502:99–101. DOI: 10.1016/j.cca.2019.12.012. PMID: 31863740.

11. Jo YA, Kim M, Kim HS, Kang HJ, Lee YK. 2013; Evaluation of the Mindray BC-6800 complete blood counts analyzer. Lab Med Online. 3:131–7. DOI: 10.3343/lmo.2013.3.3.131.

12. Briggs C, Harrison P, Grant D, Staves J, MacHin SJ. 2000; New quantitative parameters on a recently introduced automated blood cell counter - the XE 2100. Clin Lab Hematol. 22:345–50. DOI: 10.1046/j.1365-2257.2000.00330.x. PMID: 11318800.
13. Clinical, Laboratory Standards Institute. 2014. User verification of precision and estimation of bias; Approved guideline-Third edition. CLSI document EP15-A3. Wayne, PA: Clinical and Laboratory Standards Institute.
14. Clinical, Laboratory Standards Institute. 2010. Validation, verification, and quality assurance of automated hematology analyzers; Approved standard-Second edition. CLSI document H26-A2. Wayne, PA: Clinical and Laboratory Standards Institute.
15. Clinical, Laboratory Standards Institute. 2018. Measurement procedure comparison and bias estimation using patient samples, Third edition. CLSI guideline EP09c. Wayne, PA: Clinical and Laboratory Standards Institute.
16. Clinical, Laboratory Standards Institute. 2007. Reference leukocyte (WBC) differential count (proportional) and evaluation of instrumental method; Approved standard-Second edition. CLSI document H20-A2. Wayne, PA: Clinical and Laboratory Standards Institute.
17. Vis JY, Huisman A. 2016; Verification and quality control of routine hematology analyzers. Int J Lab Hematol. 38(S1):100–9. DOI: 10.1111/ijlh.12503. PMID: 27161194.

18. Mukaka MM. 2012; Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 24:69–71.
19. Nakul-Aquaronne D, Sudaka-Sammarcelli I, Ferro-Vacher C, Starck B, Bayle J. 2003; Evaluation of the Sysmex Xe-2100 hematology analyzer in hospital use. J Clin Lab Anal. 17:113–23. DOI: 10.1002/jcla.10083. PMID: 12784259. PMCID: PMC6807756.
20. Takemura H, Ai T, Kimura K, Nagasaka K, Takahashi T, Tsuchiya K, et al. 2018; Evaluation of cell count and classification capabilities in body fluids using a fully automated Sysmex XN equipped with high-sensitive Analysis (hsA) mode and DI-60 hematology analyzer system. PLoS One. 13:e0195923. DOI: 10.1371/journal.pone.0195923. PMID: 29698492. PMCID: PMC5919509.

21. Lesesve JF, Benbih M, Lecompte T. 2005; Accurate basophils counting: not an easy goal! Clin Lab Hematol. 27:143–4. DOI: 10.1111/j.1365-2257.2005.00667.x. PMID: 15784131.

22. Amundsen EK, Henriksson CE, Holthe MR, Urdal P. 2012; Is the blood basophil count sufficiently precise, accurate, and specific?: three automated hematology instruments and flow cytometry compared. Am J Clin Pathol. 137:86–92. DOI: 10.1309/AJCP19BFTHYTMORO. PMID: 22180481.

23. Kim H, Hur M, Choi SG, Moon HW, Yun YM, Hwang HS, et al. 2013; Evaluation of ABX Pentra DX 120 and Sysmex XE-2100 in umbilical cord blood. Int J Lab Hematol. 35:658–65. DOI: 10.1111/ijlh.12110. PMID: 23738834.

24. Shen Y, Cao J, Zhou Z, Wang Y, Shen Y, He J. 2019; Clinical performance evaluation of the new hematology analyzer Mindray BC-6000. Int J Lab Hematol. 41:622–34. DOI: 10.1111/ijlh.13075. PMID: 31286670.

Fig. 1
Diagram showing the flow of participants’ samples in the current study. Disease characteristics of the 92 patient samples from KUMC are noted. In the comparison study, 35 samples had partial missing data. In the flagging performance study, 21 samples were excluded due to improper operation of the instrument.
Abbreviations: AML, acute myeloid leukemia; CML, chronic myeloid leukemia; KUCH, Konkuk University Chungju Hospital; KUMC, Konkuk University Medical Center; MDS, myelodysplastic syndrome; PV, polycythemia vera.
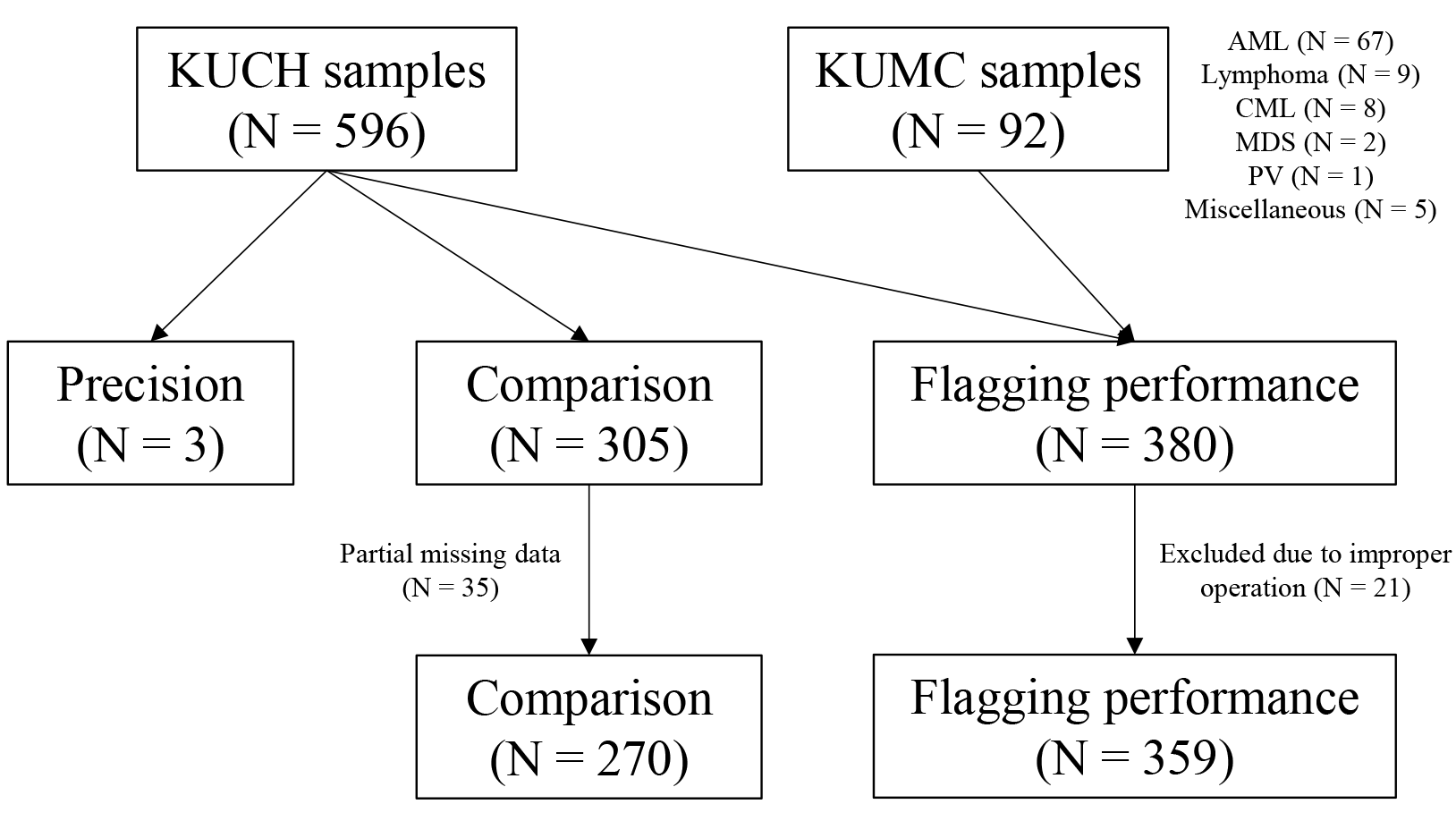
Fig. 2
Comparison results for 11 CBC parameters between BC-6200 and XE-2100 using Bland-Altman plot (N=270). Solid lines represent the mean differences, and the dashed lines represent the mean difference±1.96 standard deviations (95% limits of agreements).
Abbreviations: CBC, complete blood count; Hb, hemoglobin; Hct, hematocrit; PLT, platelets; RBC, red blood cells; RDW-CV, red blood cell distribution width-coefficient of variation; WBC, white blood cells.
Table 1
Evaluated CBC parameters and their value characteristics of the total study population (N=688)
Table 2
Within-run precision results of BC-6200 for two normal and one cytopenic sample, with the manufacturer’s requirements for each parameter
| Parameter | Sample 1 (Normal) | Sample 2 (Normal) | Sample 3 (Cytopenic) | CV (%) criteria | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||
| Mean | SD | CV (%) | Mean | SD | CV (%) | Mean | SD | CV (%) | State-of-the art* | |
| WBC (×109/L) | 6.67 | 0.11 | 1.68 | 6.74 | 0.11 | 1.57 | 2.62 | 0.07 | 2.49 | ≤ 2.5 |
| RBC (×1012/L) | 4.17 | 0.02 | 0.54 | 4.13 | 0.02 | 0.41 | 3.28 | 0.03 | 0.84 | ≤ 1.1 |
| Hb (g/dL) | 12.15 | 0.05 | 0.43 | 12.10 | 0.05 | 0.39 | 10.26 | 0.05 | 0.50 | ≤ 0.9 |
| Hct (%) | 38.12 | 0.18 | 0.46 | 37.71 | 0.17 | 0.44 | 32.19 | 0.28 | 0.87 | ≤ 1.2 |
| RDW-CV (%) | 13.42 | 0.12 | 0.92 | 13.42 | 0.13 | 0.98 | 17.06 | 0.23 | 1.33 | ≤ 2.0 |
| PLT (×109/L) | 161.10 | 2.69 | 1.67 | 161.00 | 4.40 | 2.73 | 45.60 | 1.58 | 3.46 | ≤ 3.0† |
| Reticulocytes (%) | 1.05 | 0.04 | 3.35 | 1.03 | 0.04 | 3.70 | 0.65 | 0.04 | 5.74 | ≤ 10.0 |
| Neutrophils (×109/L) | 4.31 | 0.09 | 1.99 | 4.37 | 0.06 | 1.34 | 1.12 | 0.04 | 3.87 | ≤ 2.5 |
| Lymphocytes (×109/L) | 1.73 | 0.07 | 3.99 | 1.72 | 0.06 | 3.76 | 1.22 | 0.02 | 1.94 | ≤ 3.5 |
| Monocytes (×109/L) | 0.46 | 0.02 | 5.10 | 0.46 | 0.02 | 4.44 | 0.24 | 0.02 | 7.79 | ≤ 8.5 |
| Eosinophils (×109/L) | 0.14 | 0.02 | 16.52 | 0.15 | 0.01 | 6.21 | 0.03 | 0.01 | 24.65 | ≤ 10.0 |
| Basophils (×109/L) | 0.03 | 0.01 | 24.56 | 0.04 | 0.01 | 18.00 | 0.01 | 0.01 | 51.60 | ≤ 20.0 |
*Vis et al. [17]; †≤ 4.5 for low range (~50 ×109/L) of PLT.
Table 3
Comparison of major CBC parameters between BC-6200 and XE-2100 (N=270)
Table 4
TP, FP, TN, FN, sensitivity, specificity, and efficiency results obtained from comparing BC-6200 with manual microscopy (N = 359)
| Flags | TP | FP | TN | FN | Sensitivity (%) | Specificity (%) | Efficiency (%) |
|---|---|---|---|---|---|---|---|
| Blasts | 69 | 22 | 259 | 9 | 88.5 | 92.2 | 91.4 |
| IG | 30 | 70 | 255 | 4 | 88.2 | 78.5 | 79.4 |
| AL | 3 | 87 | 268 | 1 | 75.0 | 75.5 | 75.5 |
| NRBCs | 8 | 3 | 346 | 2 | 80.0 | 99.1 | 98.6 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download