INTRODUCTION
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by the destruction of synovial joints and systemic inflammation. Initial symptoms include joint pain, fatigue, and stiffness; and if inappropriately managed, can lead to joint damage, reduced function, and impaired quality of life [
1]. The emergence of biological or targeted synthetic disease-modifying anti-rheumatic drugs (bDMARDs or tsDMARDs) have contributed to the recent improvements in the treatment of RA, and recommended treatments have evolved to focus on a targeted approach to achieve either remission or low disease activity. There has been a dramatic improvement in outcomes of RA treatment with the introduction of bDMARDs or tsDMARDs and a targeted approach [
2,
3]. However, patient-reported symptoms, such as pain and fatigue, may persist despite RA remission, with the experience of pain among the top three complaints, alongside functional disability and fatigue [
4]. Approximately 40% and 51.6% of patients treated with bDMARDs and tsDMARDs, respectively, complain of significant pain [
5]. After 6 months of the targeted approach in patients with early RA, 40.2% of patients reported unsatisfactory pain relief, which was defined as a subjective improvement <3 points on the visual analog score (VAS) for pain [
6]. The prognostic factors for achieving a satisfactory improvement in pain were symmetrical joint involvement, positivity for anti-citrullinated protein antibody (anti-CCP Ab), and fewer tender joints at baseline [
6].
This study assessed the pain prevalence in patients with RA in clinical remission (according to the Disease Activity Score of 28 joints-erythrocyte sedimentation rate [DAS28-ESR]) and analyzed the demographic and clinical characteristics of those who experienced persistent pain.
DISCUSSION
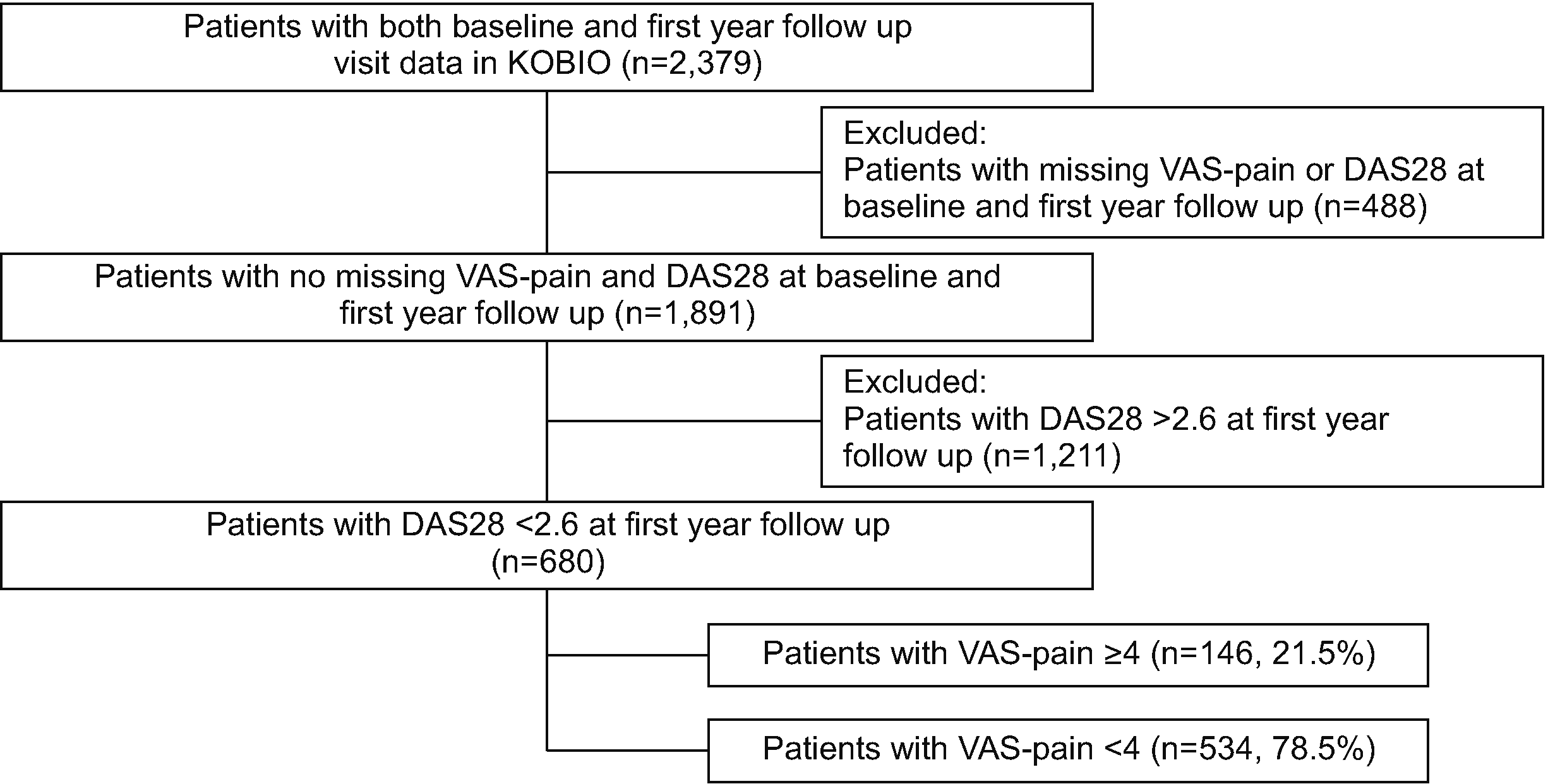
In this study, the proportion of patients who achieved DAS28-remission in the first year after using either bDMARDs or tsDMARDs was 36.0% (n=680). Among them, 21.5% (n=146) had moderate-to-severe pain, longer disease duration, and higher frequency of foot erosion, mental illness, and endocrine, renal, and neurological comorbidities than patients with a milder degree of pain. Further evaluation with multivariable regression analysis showed that the presence of foot erosions and neurological disorders were independently associated with moderate-to-severe pain in patients with RA despite clinical remission. Corticosteroid use was associated with moderate-to severe pain, in contrast, patients using JAKis and had higher ESR levels had a lesser likelihood of having moderate-to-severe pain.
Pain is generally considered a surrogate marker of inflammatory disease activity in RA [
10]; and is not only the single dominant determinant of patient’s GA, but also a prominent component of the ACR/EULAR criteria for remission [
11,
12]. In our study, multiple regression data showed that the presence of foot erosions was a predictor of moderate-to-severe pain, although ESR levels had a lesser likelihood of eliciting moderate-to-severe pain. This result may be related to the fact that DAS28 does not include the feet, and indicates that disease control beyond the 28 joints is also important for proper pain control in patients with RA. In terms of the inverse association between ESR and moderate-to-severe pain, we can consider the following. First, it seems questionable whether the clinical implication of ESR in predicting pain is meaningful when the mean levels of ESR in both groups were below 10 mm/hr. Second, corticosteroid use, which was an independent factor for predicting moderate-to-severe pain, could have affected the ESR value leading to the inverse association. And third, tocilizumab was prescribed more frequently in patients with moderate-to-severe pain (56.2% vs. 39.7%), which could have resulted in a lower mean level of ESR at remission than patients with a milder degree of pain.
Persistent pain is not always relative to inflammatory conditions, whereby persistent pain in RA is a complex and multifactorial phenomenon linked to central pain mechanisms such as central and peripheral sensitization as well as peripheral (neurogenic) inflammation [
5,
13]. A previous study identified clusters of patients with RA with different causes of pain, treatment options, and prognoses, and found three subgroups of patients based on pain, inflammation, fatigue, and psychological distress [
14]. One subgroup showed minimal inflammation but high levels of pain, psychosocial distress, and fatigue. Indeed, the prevalence of comorbid fibromyalgia in populations with RA is significantly higher than that in the general population, with pooled prevalence estimates of 18%~24% [
15]. Central sensitization and fibromyalgia may explain the disconnect between persistent pain and improved inflammation in pain in RA [
15,
16]. However, since the KOBIO study did not accurately collect data regarding fibromyalgia, anxiety, and depression, this study could not evaluate the association between pain and these components.
This study showed that the moderate-to-severe pain population had a higher frequency of comorbidities, such as neurological, endocrine, and renal disorders, than those with a milder degree of pain despite clinical remission. In the univariable regression analysis, comorbidities, such as neurological, endocrine, and renal disorders, were associated with moderate-to-severe pain in patients with RA. Further evaluation with multivariable regression analysis showed that neurological disorders with an OR of 3.93 were independently associated with moderate-to-severe pain in patients with RA despite clinical remission. These results are consistent with a previous study that showed that patients with chronic pain had a higher prevalence of comorbidities [
17]. A significantly higher prevalence of comorbidities and up to four times the OR of multimorbidity in the chronic pain group compared to those in the non-chronic pain group was demonstrated [
17]. Furthermore, a recent study evaluated contributing factors in difficult-to-treat patients with RA and showed that several contributing factors, such as limited drug options related to adverse events, comorbidities, and concomitant fibromyalgia, were independently associated with difficult-to-treat RA [
18]. Therefore, our results, as with previous studies, suggest that it may be important to manage comorbidities as well as treat disease activity for pain control in patients with RA. However, further research to support that pain in patients with RA improves when comorbidities are well-managed is needed.
Immune cells and their mediators, including pro-inflammatory cytokines, have been identified as important contributors to various types of pain [
19]. RA treatment, including both bDMARDs and tsDMARDs with cytokine-blocking effects, may have pain-reducing effects independent of their anti-inflammatory effects [
20,
21]. Recent reports have shown that patients with RA treated with JAKis significantly achieved improvements in pain over those treated with anti-TNF, although standard clinical measures and markers of inflammation were similarly improved in both treatment groups [
21]. In this study, there was a difference in the proportions of bDMARD or tsDMARDs prescribed to patients with moderate-to-severe pain and those with mild or no pain. Considering the proportion of prescribed DMARDs for mild and moderate-to-severe pain and that JAKis were observed on multivariate analysis to have a lesser likelihood to eliciting moderate-to-severe pain despite remission status, it is suggestive of different mechanisms for each anti-cytokine drug resulting in different pain control, and that even if DAS28-remission is reached, pain control may differ depending on the drug used. However, in-depth etiopathological mechanisms or comparative clinical studies that identify the effect of both bDMARDs and tsDMARDs on pain in RA have not been conducted, and further research is needed to support this suggestion.
This study has some limitations. First, as fatigue, anxiety, and depression were not quantitatively assessed in the KOBIO registry, we could not analyze the relationship between pain and these conditions. Second, we could not assess how fibromyalgia affected pain in these populations. Third, this study was cross-sectional and did not assess pain fluctuations over time. However, this study presents real-world data in patients with RA treated with both bDMARDs and tsDMARDs, and data on factors influencing pain were evaluated in patients with RA in remission. Furthermore, this study recommends the evaluation of the cause of pain from various angles in patients with RA and formulation of a new therapeutic plan for these patients. Further prospective studies from multiple centers will provide a better understanding of pain in patients with RA and development of therapeutics targeting pain.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download