Abstract
Background
Use of opioids for chronic intractable pain is increasing globally, and their proper use can improve patients’ quality of life. In contrast, opioid use disorders, such as abuse or addiction, caused by prescribing opioids, are a worldwide issue. This study aimed to understand current opioid prescribing patterns and pain physicians’ experiences with opioid use in South Korea.
Methods
Pain physicians in 42 university hospitals in South Korea were asked to complete anonymous questionnaires regarding opioid prescriptions.
Results
A total of 69 surveys were completed. Most pain physicians started prescribing opioids at a pain score of 7/10 and aimed to reduce pain by 50%. Most physicians (73.1%) actively explained the prescribed medications and possible side effects, and 61.2% of physicians preferred the prescription interval of 4 weeks. Immediate-release opioids were the most popular treatment for breakthrough pain (92.6%). The most common side effect encountered by physicians was constipation (43.3%), followed by nausea/vomiting (34.3%). Of the physicians, 56.5% replied that addiction and misuse prevalences were less than 5%. However, the most concerning side effect was addiction (33.0%).
Conclusions
The survey results showed that the prescribing patterns of pain physicians generally followed Korean guidelines. Physicians were most interested in the safety and effectiveness of opioid prescriptions. They were most concerned about respiratory depression and abuse or addiction. A significant number of physicians agreed that the NHIS regulations needed improvement for patient convenience and safe and effective treatment, though there were pros and cons of the NHIS restrictions on prescription conditions.
Effective pain treatment can lead to a productive life and improve the quality of life (QOL) for many patients [1,2] because patients with chronic non-cancer pain (CNCP) reportedly have a health-related QOL as poor as those dying of cancer [3]. Chronic pain has been defined in several ways, for example, “pain that lasts for 6 months after injury and beyond the usual or reasonable time of healing [4], or “considering pathological degree, pain that lasts for at least 3 months or longer than expected” [5,6]. When considering treatment for patients with CNCP, the optimization of non-opioid pharmacotherapy and non-pharmacologic therapy is recommended [7]. However, for those who have persistent problems despite optimized non-opioid treatment, opioids should be added rather than continuing therapy without opioids [8]. Opioids are typically reserved for moderate to severe pain that cannot be alleviated with acetaminophen or nonsteroidal anti-inflammatory drugs [9].
As patients and the medical community become aware of the need for pain control, physicians have more opportunities to provide opioid prescriptions [10]. A recent study conducted in South Korea showed a dramatic increase in opioid use [11,12]. As effective pain relief is an essential element of current health care, opioids play a critical role in managing CNCP. However, the adverse effects of opioid addiction have escalated sharply [13], requiring a careful prescription to suppress potential harms [14]. However, the opioid prescription pattern for CNCP in Korea appears different from that in Europe and America [8,15]. Despite the increased consumption of opioids in South Korea, pain physicians tend to have opiophobia when it comes to prescribing opioids [16]. Other studies have shown that many physicians have a passive attitude toward prescribing opioid analgesics because of the risk of opioid use disorder [17,18].
The Korean National Healthcare Insurance Service (NHIS) restricts the quantity and duration of prescribed opioids, and the narcotic information management system operates under government control [11]. If regulations to prevent opioid abuse and addiction are too strict, pain control may be insufficient [19]. In contrast, for early users, initiation or escalation to higher-dose opioids may lead to long-term use. Physicians’ prescription behavior is related to increased opioid prescriptions and long-term opioid use [20]. The increase in opioid abuse can be attributed to physician prescriptions [21]. Before initiating opioids, physicians should set treatment goals with patients and consider how opioids will be discontinued if the benefits do not outweigh the risks [22,23]. Experts from the Opioid Research Group have prepared guidelines for prescribing opioids for CNCP in Korea by reviewing various research data-related issues in Korea and several other countries in 2017. Guidelines should be continuously modified in light of new clinical evidence. Physicians’ experiences and opinions should also be considered in the modification of guidelines since they have been working with the guidelines in the field.
Therefore, we conducted the survey to review the latest trends in prescribing opioids and to collect the physicians’ opinions regarding the guidelines. This survey investigates the starting point, pattern, and treatment goal of opioid prescriptions; opinions about the limited period and dose of opioid prescription by the NHIS; and physicians’ experiences with opioid medication in South Korea.
The requirement for ethical approval for this study was waived by the Institutional Review Board of Konkuk University Medical Center (IRB file No. 2019-12-014). The survey was conducted by the Public Relations Committee of the Korean Pain Society between September and October 2018. Based on the results of this anonymous survey, this manuscript was drafted in 2022.
Physicians who worked at pain clinics in 42 university hospitals in Korea were asked to complete anonymous questionnaires regarding opioid prescription. Physicians were informed that the survey aimed to investigate the actual situation and patterns of opioid prescription by physicians in Korea and that there would be no personal advantage or disadvantage, regardless of whether they participated in the survey. Physicians who agreed to participate in the study were surveyed, and those who did not agree to participate were excluded. The questionnaire consisted of the following questions (Appendix): (1) general information such as experience in pain clinics, diseases requiring frequent opioid prescription, and the proportion of patients prescribed opioids; (2) opioid prescription behavior (e.g., pain score for the first opioid prescription, pain relief goals, whether to explain the side effects in advance, preference for combination opioids, prescription intervals, and treatment for breakthrough pain); (3) opinions regarding sanctions imposed by the NHIS on prescription intervals and dose; and (4) experience with opioid medication (e.g., side effects and patient compliance, abuse, and addiction) investigated using a specially designed survey.
A total of 69 pain physicians from 42 university hospitals completed the questionnaire. Table 1 summarizes the survey’s basic statistics: the practice period of physicians who responded to the questionnaires, diseases requiring frequent prescription of opioids, and proportion of patients prescribed opioids. More than half of the respondents (53.6%) had pain clinic careers for more than 10 years, and only one physician did not prescribe opioids (1.5%). Cancer pain (31.3%) and complex regional pain syndrome (CRPS) (29.7%) were representative diseases for which physicians frequently prescribed opioids.
Demographic data
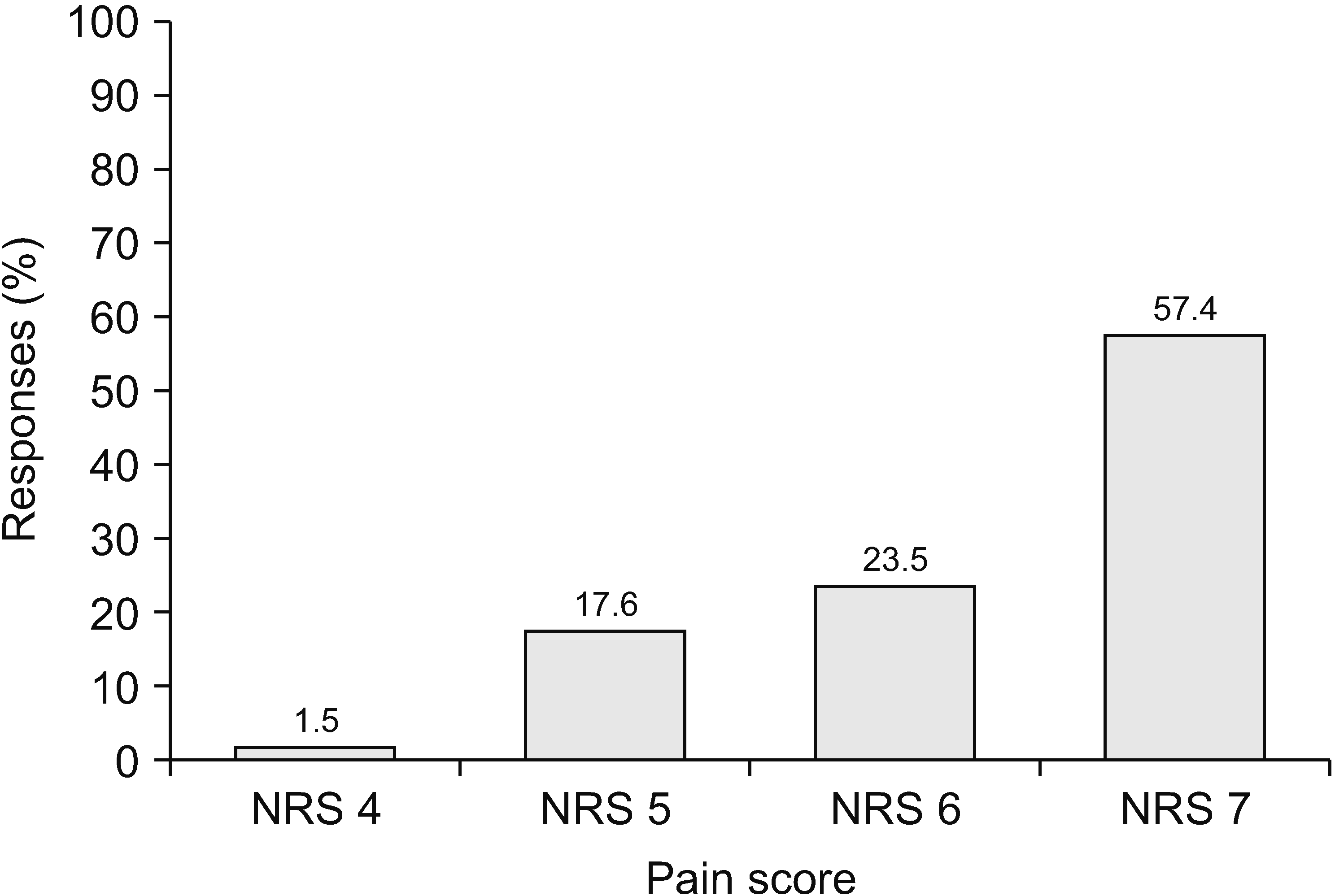
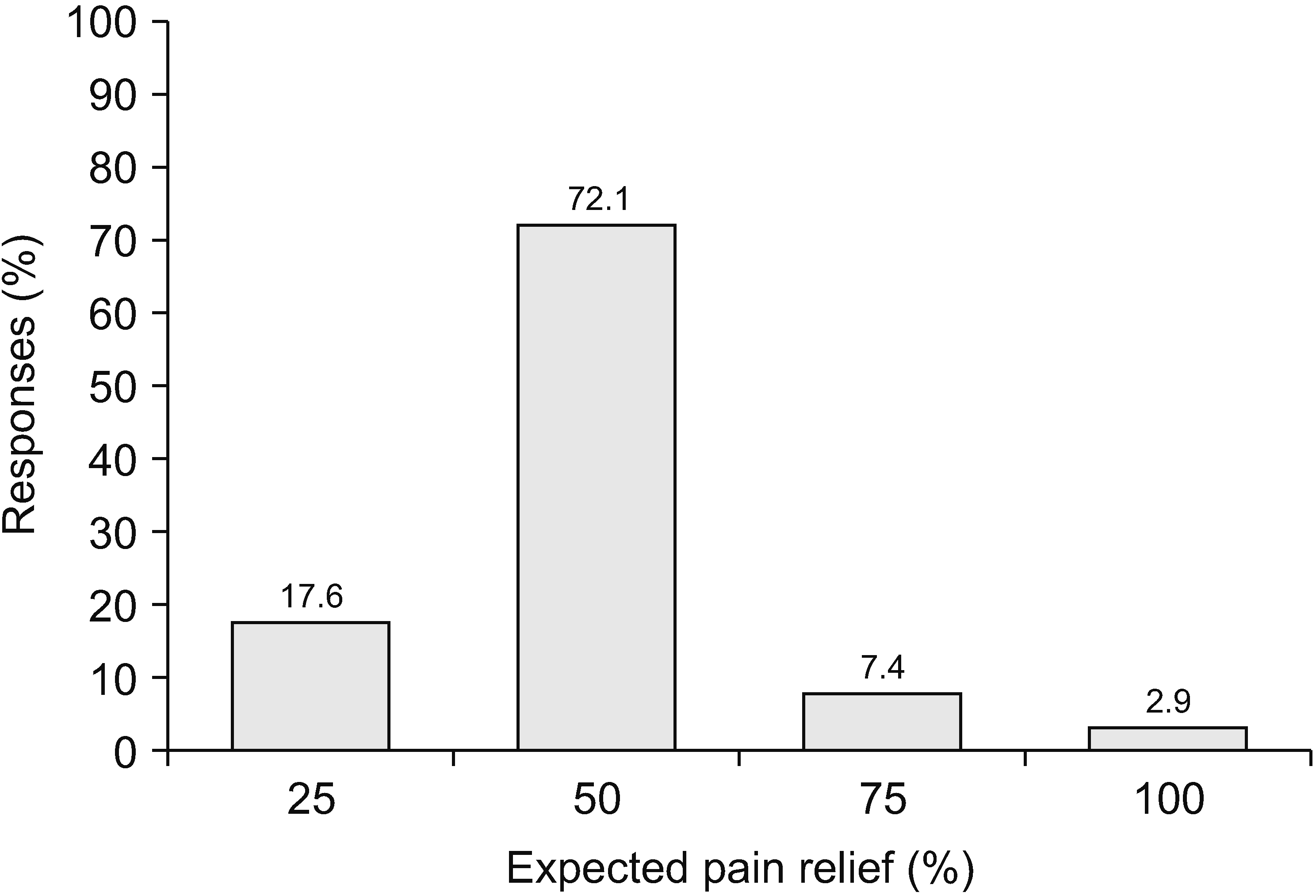
Sixty-eight physicians (98.6%) with experience prescribing opioids, excluding one who said they did not prescribe drugs, were asked about the patients’ pain scores when they started to prescribe opioids. Fig. 1 shows that 57.4% of physicians decided to prescribe opioids to patients with a pain score > numeric rating scale (NRS) 7. The opioid prescription prevalence decreased according to pain score; for example, 23.5% of physicians began to prescribe opioids when the pain score was more than NRS 6 and 17.6% for a pain score more than NRS 5. When prescribing opioids, most physicians (72.1%) aimed to reduce pain by 50% and 17.6% of physicians by 25%, as shown in Fig. 2. In addition, 2.9% of physicians expected to eliminate pain.
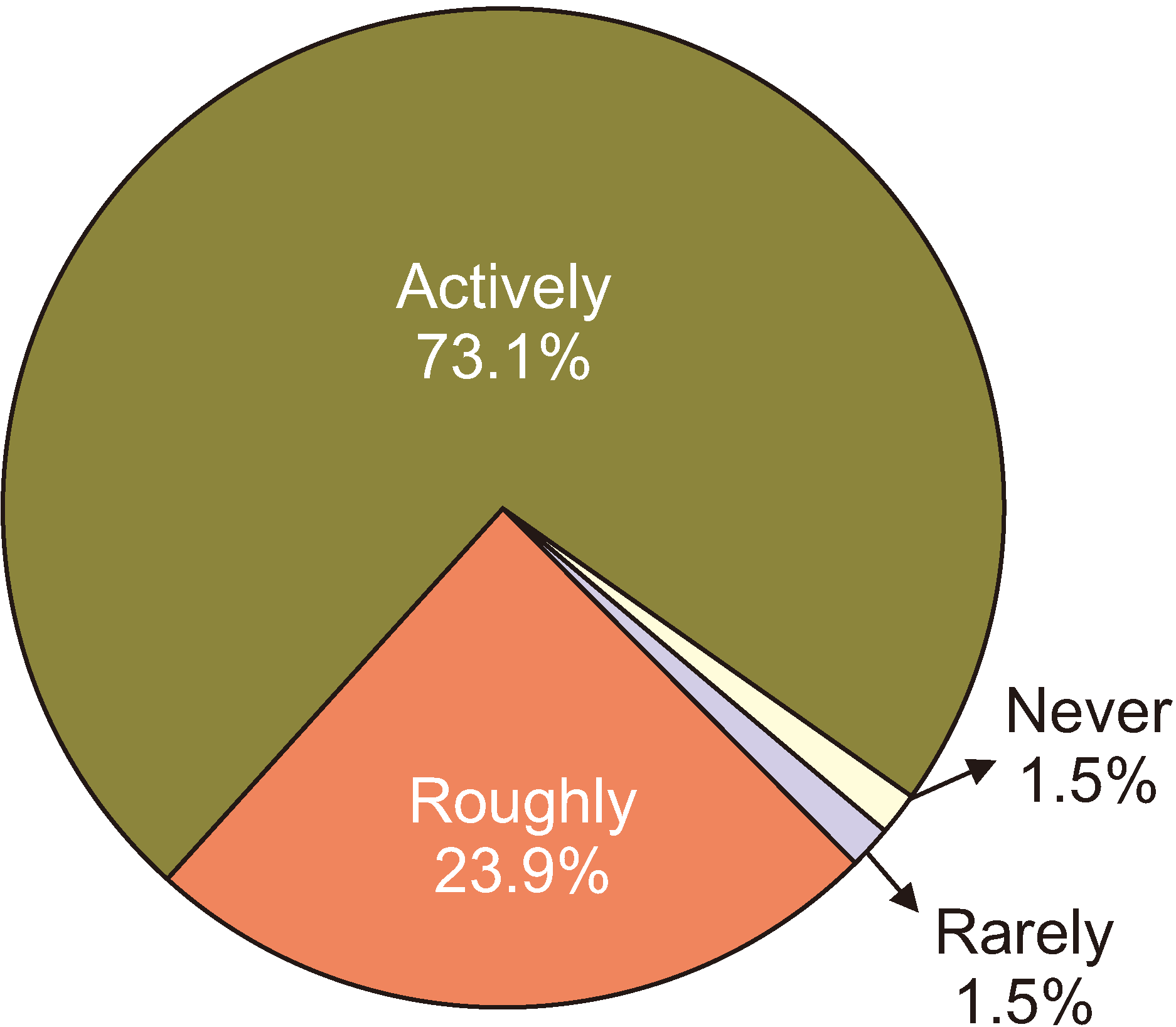
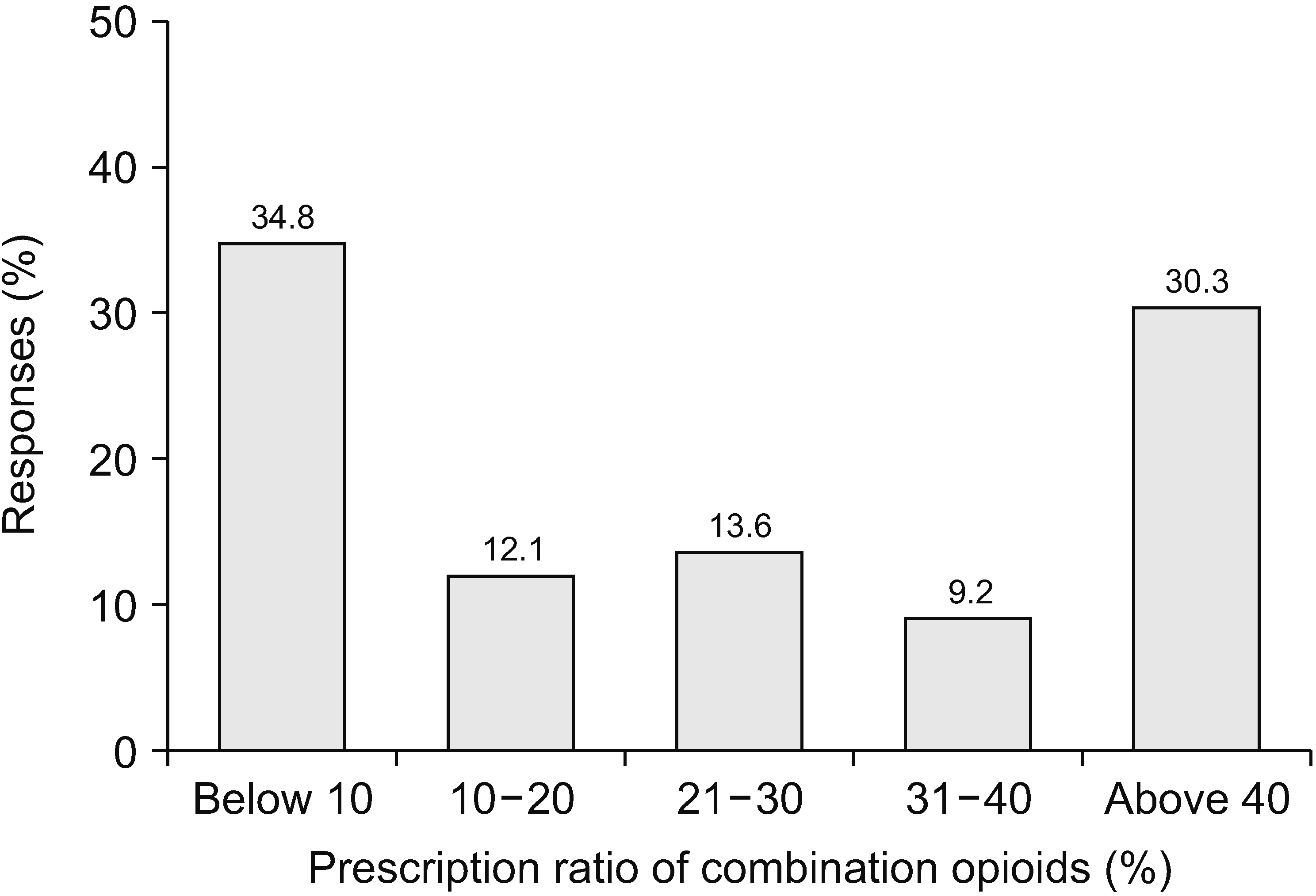
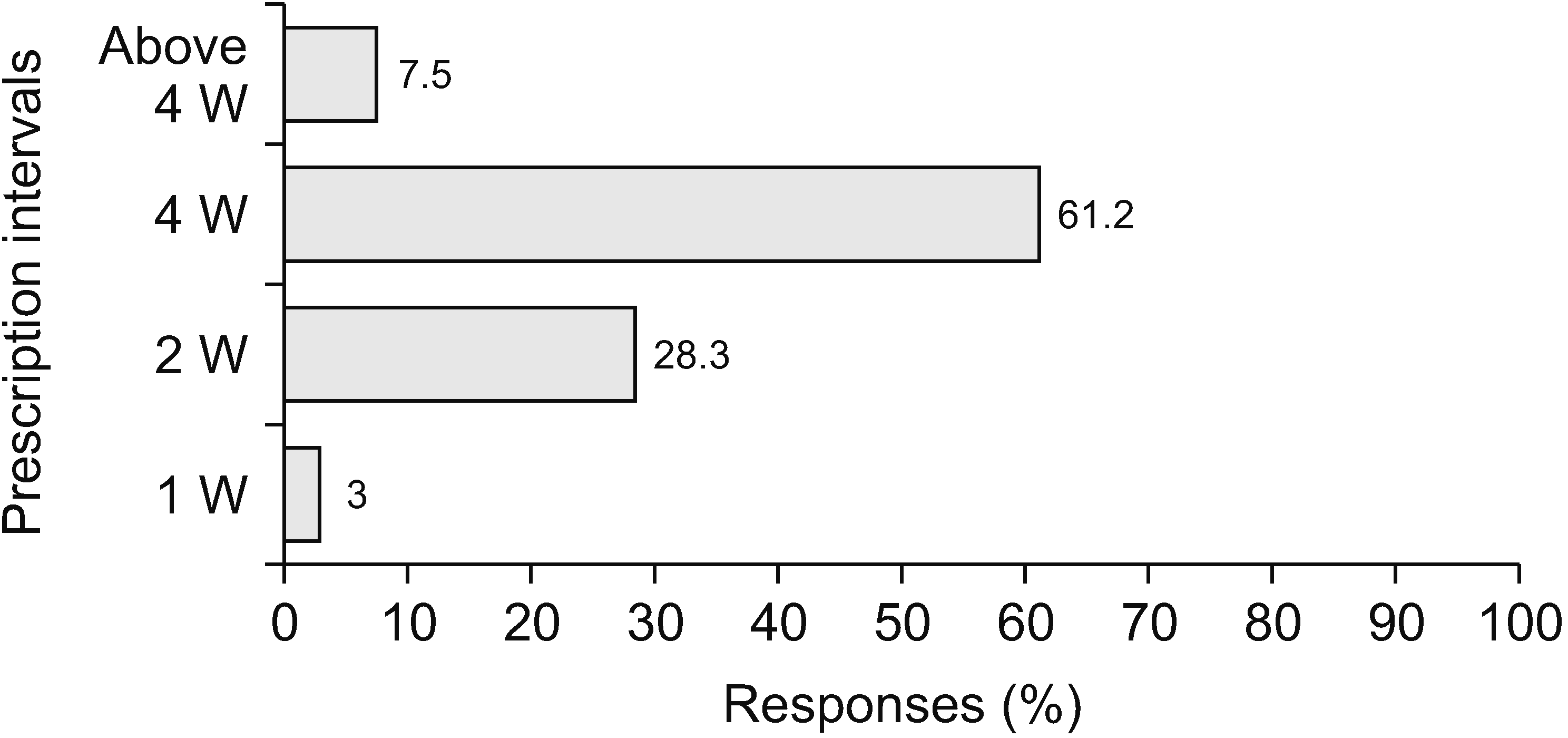
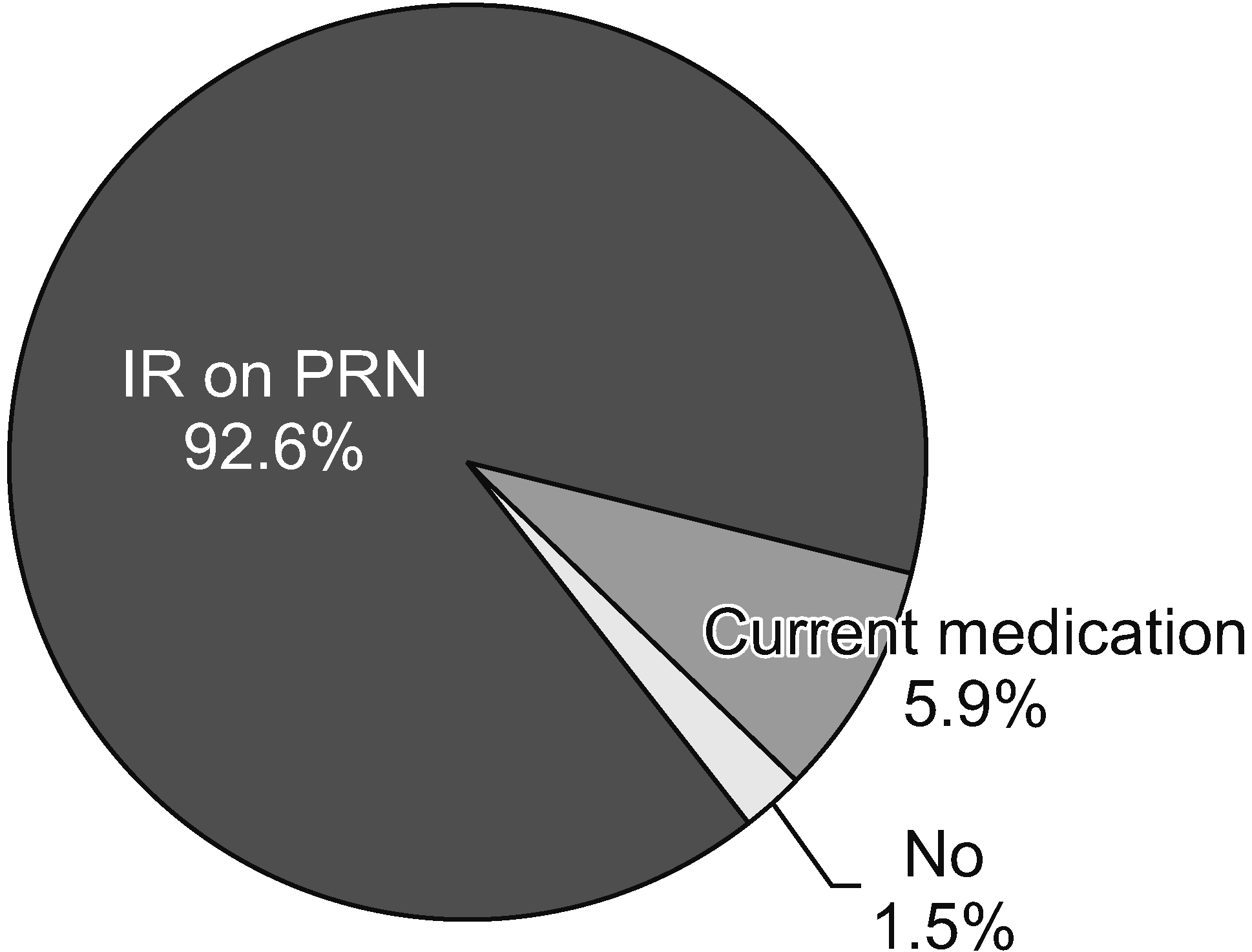
When physicians prescribed opioids, most respondents (73.1%) actively explained their prescription of opioids and the possible side effects. Only 1.5% of respondents did not explain their opioid prescriptions, as shown in Fig. 3. Fig. 4 illustrates two extreme prescription behaviors for combination opioids; for 34.8% of respondents, combination opioids accounted for less than 10% of their total opioid prescriptions, while 30.3% preferred combination opioids (more than 40% of the total opioid prescriptions). Most physicians (61.2%) preferred the prescription interval to be 4 weeks (Fig. 5). Patients often complained of breakthrough pain, and Fig. 6 shows how the physicians responded to it. Immediate-release (IR) opioids were the most popular treatment (92.6% of respondents), and an additional dose of current drugs was the second most favored treatment (5.9% of respondents).
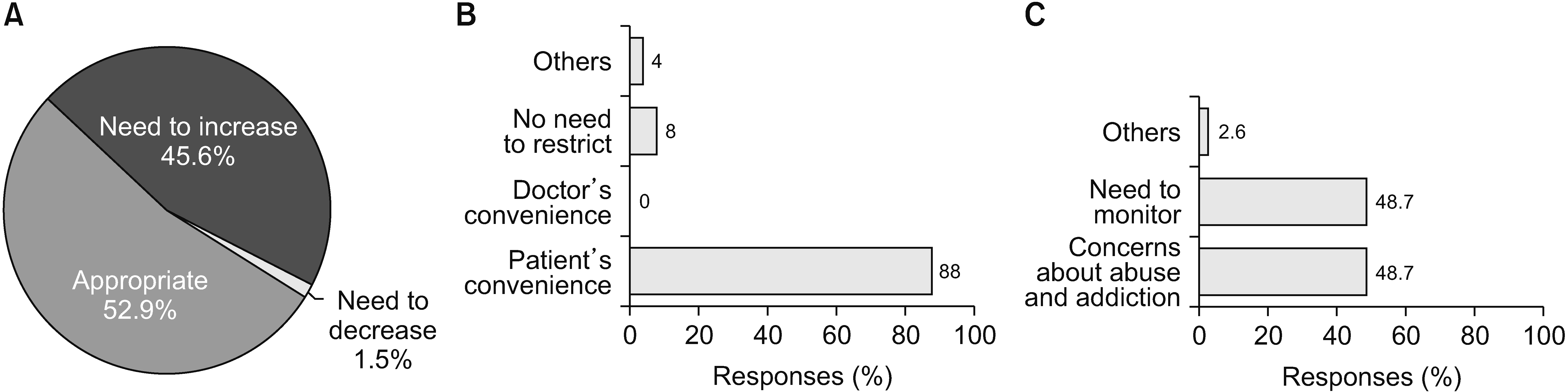
Regarding the 30-day limitation, 52.9% of 68 respondents said it was reasonable, 45.6% thought the period should be extended, and one physician (1.5%) believed that the period should be shortened (Fig. 7A). Fifty respondents provided a reason for increasing the number of limited prescription days (Fig. 7B). Approximately 88% said it was for the patients’ convenience, and 8% thought there should be no limit on the prescription interval because they believed opioids were safe. Thirty-nine respondents provided a reason for decreasing the number of prescription days (Fig. 7C). Of the respondents, 48.7% reported abuse or addiction. Another 48.7% attributed it to monitoring efficacy and adverse reactions.
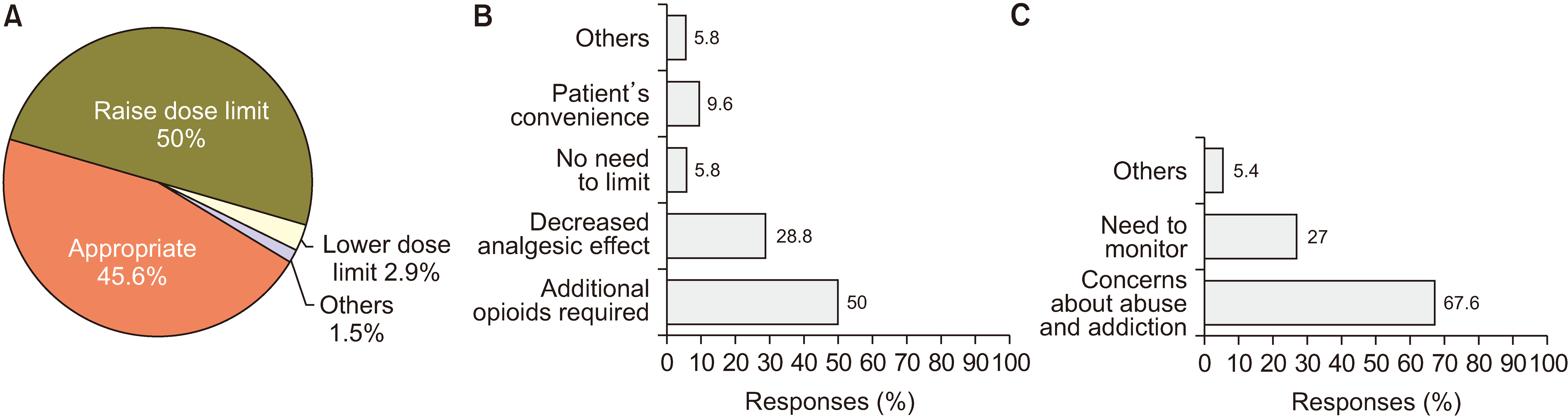
The NHIS imposes restrictions on the dose of opioids for noncancer pain. Physicians’ views on this dose limit were collected and analyzed (Fig. 8). Among the 68 respondents, 50% wanted the dose limit raised, whereas 45.6% said that the current limitation was appropriate. Fifty-two physicians provided a reason for increasing the dose limit, and 50% of physicians pointed out the need for another drug resulting from the limited opioid dose. This was ascribed to the decrease in analgesic effect due to opioid tolerance (28.8%). Only 5.8% thought dose limits were unnecessary because opioids are safe.
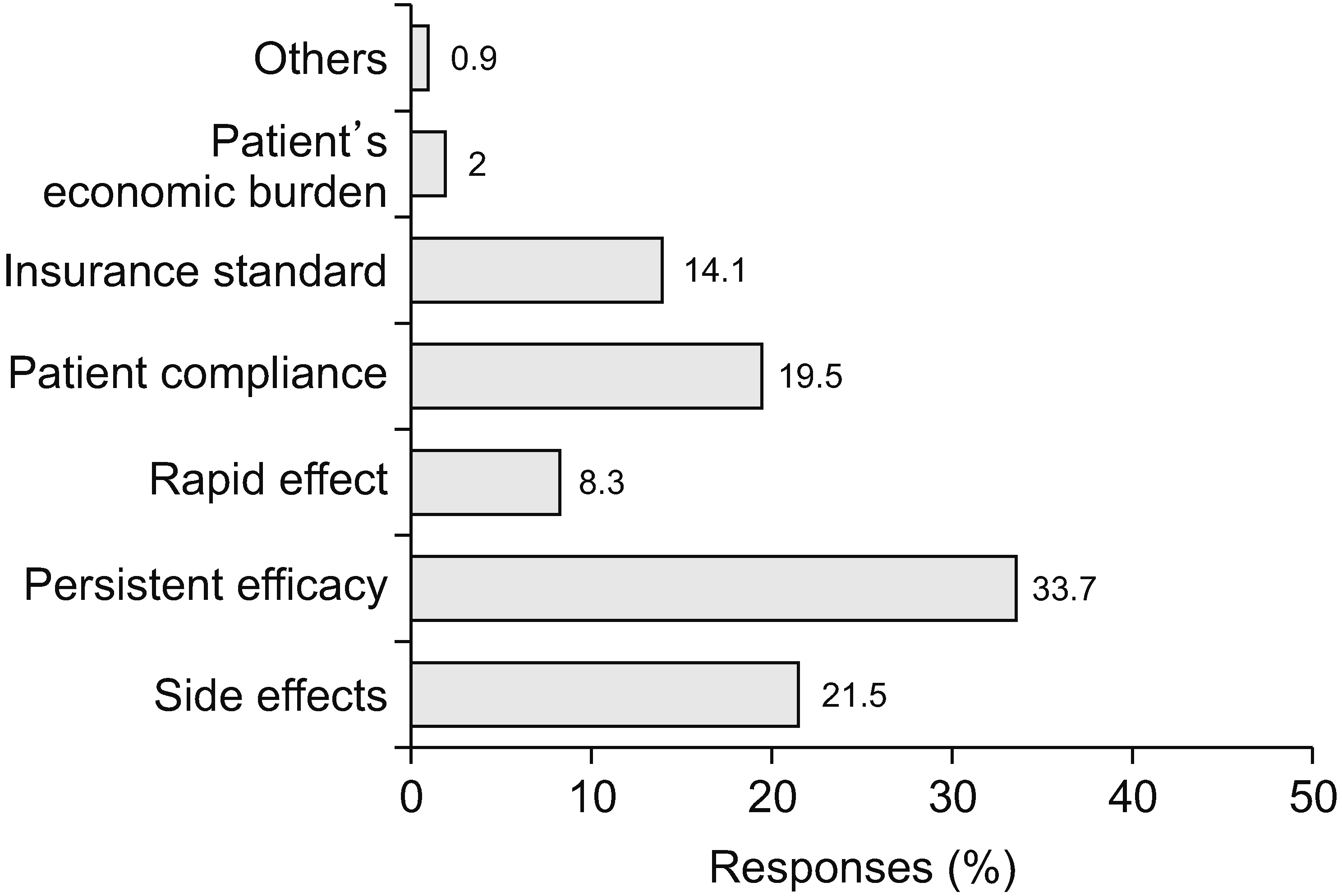
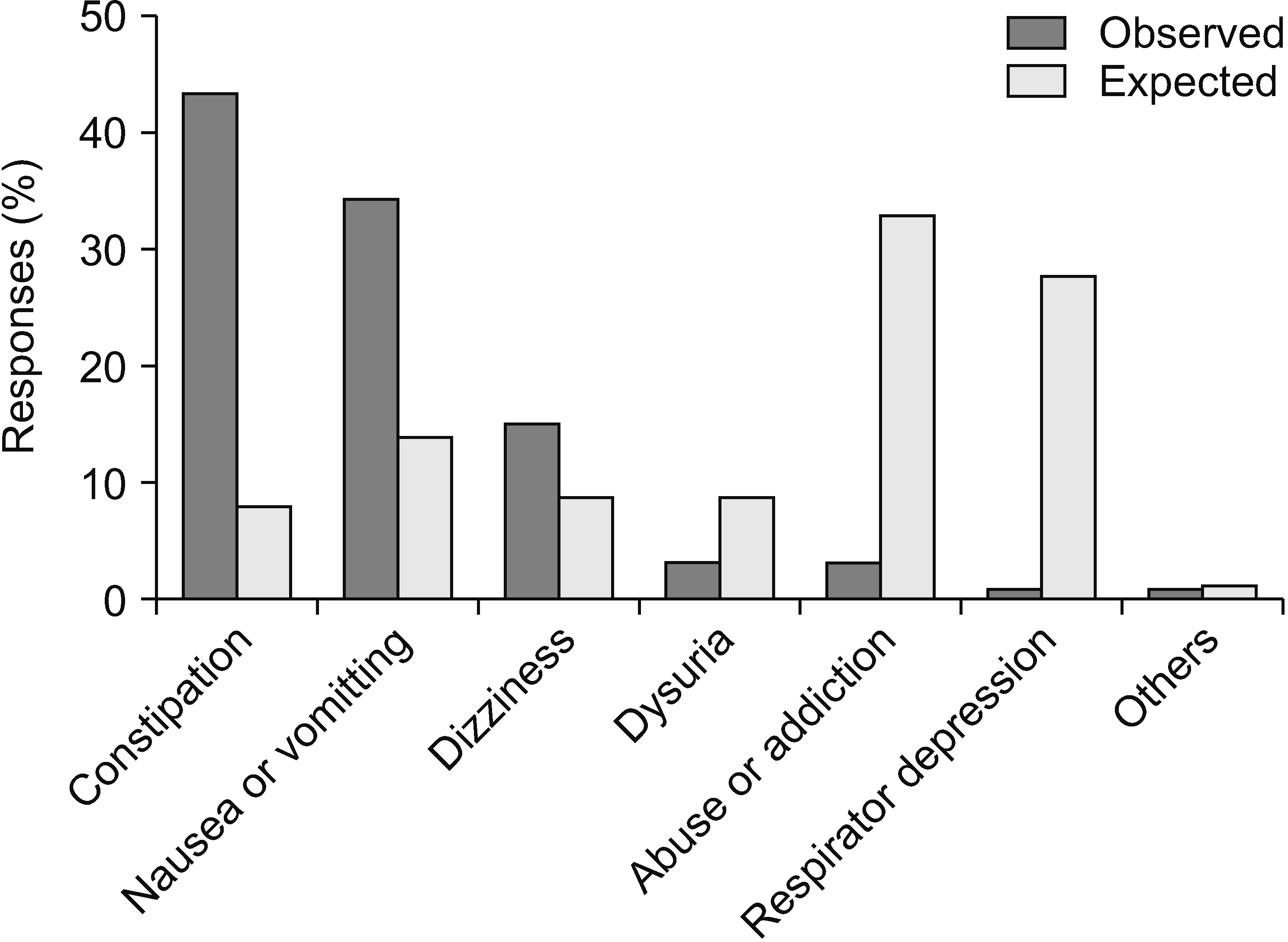
Factors considered while prescribing opioids (Fig. 9) were persistent efficacy (33.7%), side effects (21.5%), patient compliance (19.5%), insurance standards (14.1%), rapid effect (8.3%), and the patient’s financial burden (2%). The most common side effect of opioid medication was constipation (43.3% of the total 134 responses), followed by nausea/vomiting (34.3%), and dizziness (14.9%). Dysuria, addiction, and misuse remained at the bottom of the ranks (3.0% each). However, physicians were most concerned about addiction and misuse of opioids (33.0% of the total 115 responses). Respiratory depression or loss of consciousness (27.8%) were observed. Nausea/vomiting (13.9%), dizziness (8.7%), dysuria (8.7%), and constipation (7.8%) were relatively minor concerns for physicians (Fig. 10).
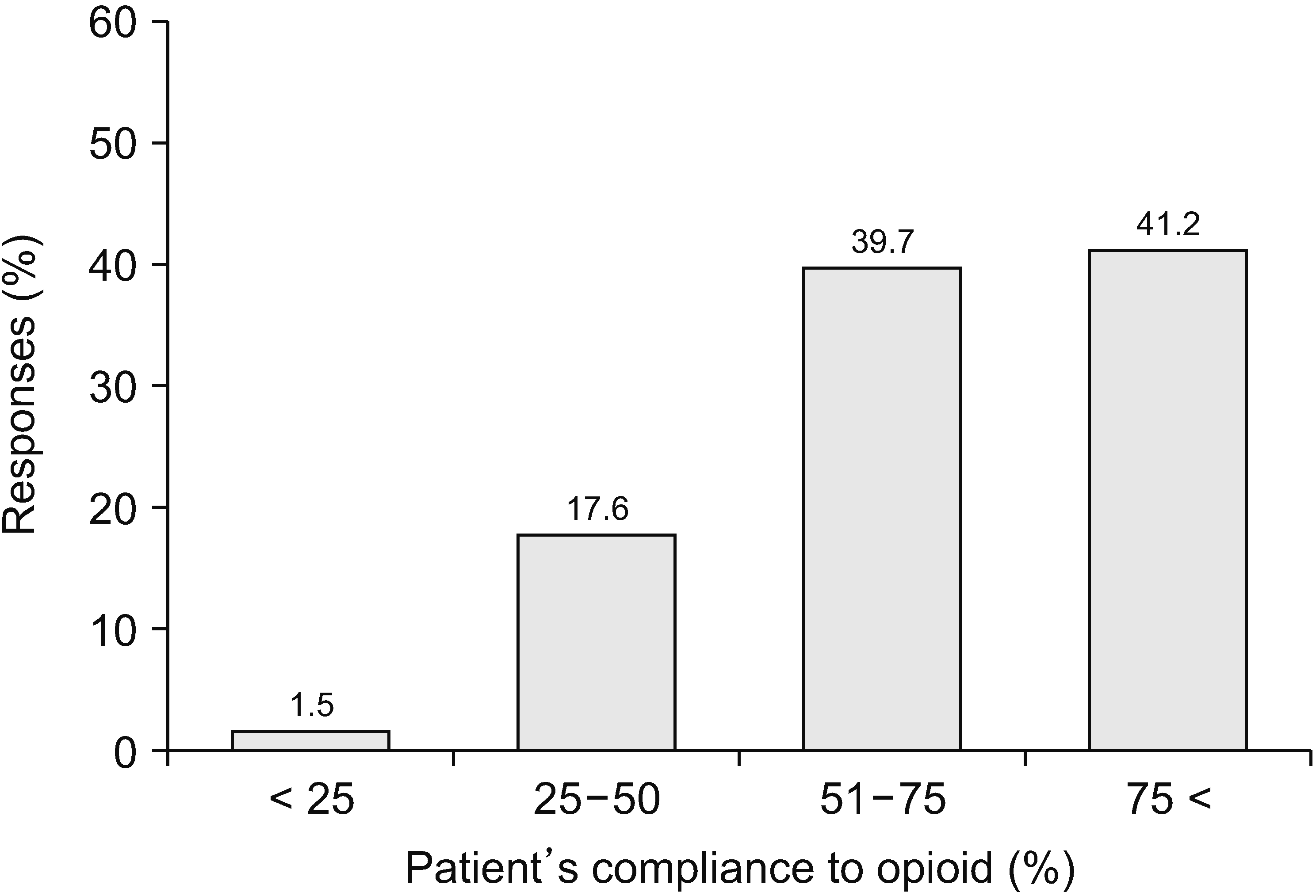
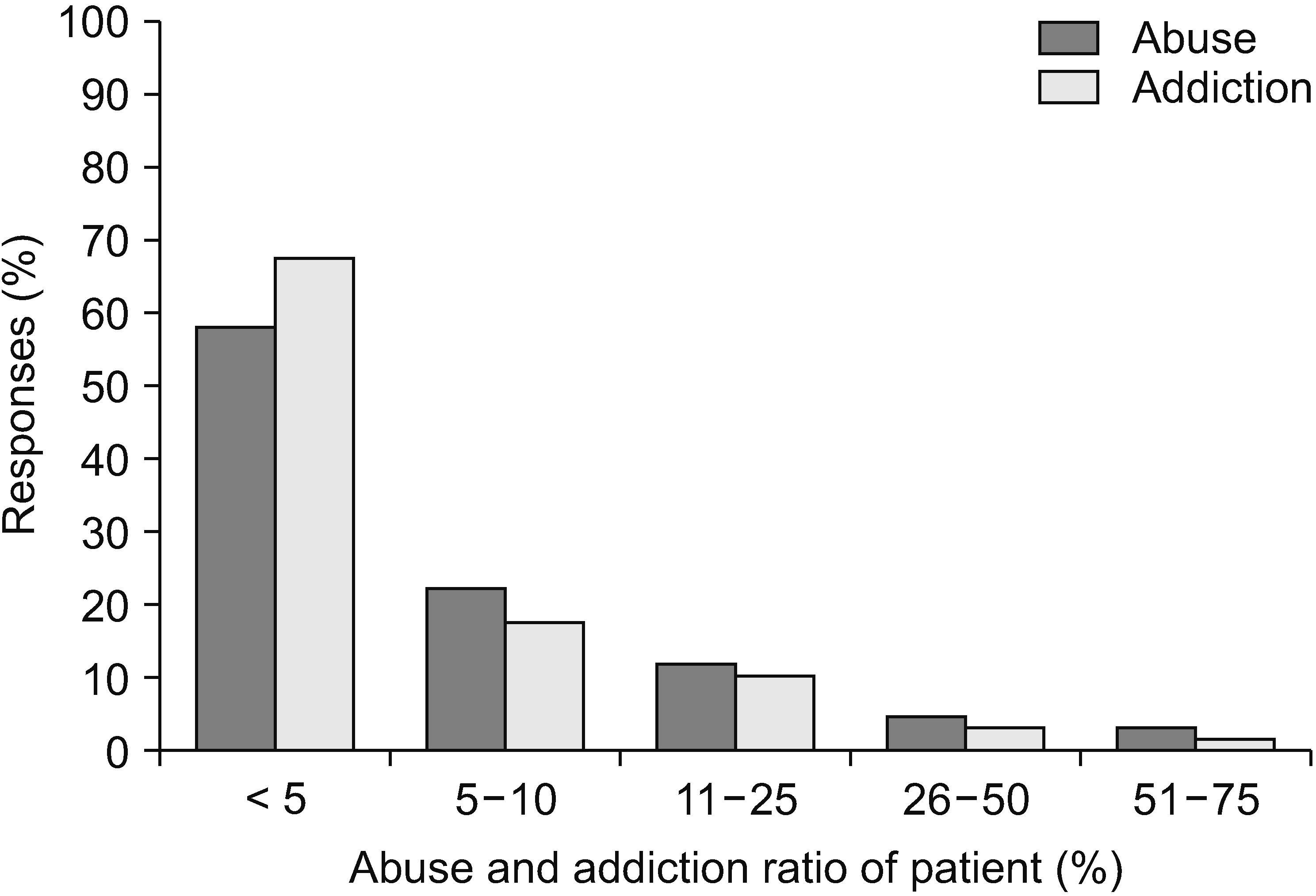
Patient compliance with opioid medication was high: compliance > 75% was 41.2%, 51%–75% was 39.7%, 26%–50% was 17.6%, and < 25% was only 1.5% (Fig. 11). Regarding the percentage of misuse or abuse, 56.5% of 67 physicians replied that it was less than 5% (Fig. 12). Of 67 respondents, 66.7% reported that only 5% of their patients were addicted. Three physicians (4.5%) answered that more than 26% of their patients were addicted (Fig. 12).
Several studies have shown that chronic pain is prevalent in 2%–40% of the adult population [4]. In the United States, one-third of individuals reported experiencing chronic pain [13]. The high medical costs of treating chronic pain and low labor productivity have resulted in a national economic burden. Moreover, pain-related medical expenses are reportedly greater than those for heart disease, cancer, and diabetes in the United States [24]. The effective treatment of chronic pain enables many patients to lead productive lives and improve their QOL.
Although opioids are not the most effective drugs for CNCP, their administration to patients with chronic pain has drastically increased [23,24]. Opioid prescriptions in Korea are steadily increasing, although they are still fewer than the average in Organization for Economic Cooperation and Development countries [15,25]. Table 1 lists diseases requiring frequent prescription of opioids in pain clinics; cancer pain (31.3%) ranked as the top single condition requiring opioid prescription, but the sum of non-cancer pain treatments (around 69%) outnumbered cancer pain as coinciding with global trends of increased opioids use in CNCP [26,27].
Oral opioids are reportedly effective in the treatment of CNCP in patients without a history of mental illness, and the possibility of addiction is low [22]. Opioids for CNCP [28] include chronic low back pain [28], osteoarthritis, and neuropathic pain [29]. Opioids are also continuously prescribed to patients with cancer [30] and postoperative pain [31]. In patients with fibromyalgia, a common pain condition, only tramadol is of modest benefit; however, strong opioids (oxycodone or morphine) are not recommended [32]. In this study, common CNCP diseases with frequent opioid prescriptions were CRPS, postherpetic neuralgia (PHN), and spinal/joint disease. In previous multicenter study [33], 80.3% of CRPS patients had been consuming opioids. The severe pain of CRPS can be related to the high prescriptions of opioids.
A randomized controlled trials (RCT) meta-analysis in patients with CNCP demonstrated that the effect of opioid use was statistically significant; however, improvement in physical function and pain were small and showed short-term efficacy [7]. Moreover, evidence of its long-term effectiveness is lacking [7,34]. In contrast, a national poll of long-term opioid users in the United States revealed improvements in physical function and significant differences in life. It showed that opioids reduced pain, and that the users’ QOL was better than that of opioid-free patients [35]. Amid this controversy, opioids may be viewed as a second- or third-line treatment method when all other treatment methods are unsatisfactory. If the physician decides to start opioid therapy, opioid analogs (codeine or tramadol) are suggested as first-line opioids for mild-to-moderate CNCP. The use of more potent opioids (morphine, oxycodone, and hydromorphone) may be considered from the beginning as first-line opioids for patients with severe CNCP [8,14].
As shown in Fig. 1, 57.4% of pain physicians started prescribing opioids to patients with NRS pain scores ≥ 7. Fig. 1 also shows that all physicians began to prescribe opioids to patients with NRS pain score > 4. A review article summarizes that among patients with non-cancer pain, NRS scores of 4 and 6 define mild-to-moderate and moderate-to-severe pain, respectively. It can be used as a guide for selection and initiation of pain treatment [36].
Clinically meaningful improvement has been defined as a 30% improvement in scores for both pain (approximately 2 points on an 11-point scale [14]) and function [23]. In addition, the Korean guidelines recommend that pain relief and functional status improvement be at least 30% without adverse consequences [8]. In this survey, 72.1% of physicians aimed to reduce pain by 50%, and 82.4% of physicians aimed to reduce pain by 50% or more; however, only 17.6% of physicians expected a pain relief of 25%. As shown in Fig. 2, 2.9% of physicians hoped to resolve the pain altogether. It appears that expectations were higher than the standards recommended in the guidelines. Physicians should emphasize that elimination of pain is unlikely, and complete improvement of chronic pain by opioids is limited [14].
Recently, the importance of informing patients of opioid prescriptions and explaining side effects before prescription has been highlighted. Several medical litigation cases in Korea dealt with plaintiffs’ claims of physicians violating explanation obligations in advance [37]. In this survey, 73.1% of physicians believed that they gave an active explanation to patients, and 23.9% thought that they gave a rough description. In other words, 97% of physicians seemed to explain opioid prescriptions and their side effects in advance. Only 1.5% of physicians confessed that they did not. In another survey of Korean patients, 7.2% insisted that they did not receive any explanation regarding opioids from physicians [38]. There was a gap between the patient’s view and that of the physicians on how faithfully the physicians did their duty of advance explanation. This gap implies that physicians must provide an explanation from the patient’s point of view and obtain informed consent. Physicians should discuss opioid therapy periodically and regularly with patients [23].
Pain physicians should prescribe a single opioid analgesic and titrate it to a stable dose for cancer pain [39]. There is little evidence to support the practice of combining different opioids [14]. If pain is not adequately improved over a certain period of time, another opioid may be added in cooperation with a pain physician according to the Korean guidelines [8]. We investigated the rate of prescription of combination opioids and found that 34.8% of physicians prescribed combination opioids in fewer than 10% of their prescriptions, while 30.3% prescribed them more than 40% of their prescriptions (Fig. 4). We cannot determine which behavior is preferred or better because prescription details, such as the composition of combined drugs, are unknown and require further investigation. Combination opioid pills might improve analgesic efficacy over existing analgesia [40], however, physicians should maintain drug prescriptions at a lower dose and prescribe short- or long-acting opioid combinations with extreme caution.
Before initiating opioids, physicians should establish treatment goals for patients and consider how opioids will be discontinued if the benefits do not outweigh the risks. Physicians must prescribe opioids according to their best judgment, and it is essential to establish an appropriate goal, even if it goes against the wishes of patients [14]. When initiating opioid therapy for chronic pain, physicians should evaluate the benefits and harm to patients within 1–4 weeks of initiating opioid therapy for chronic pain or of dose increase [8,23]. Moreover, physicians should evaluate continued therapy with patients every 3 months or more frequently. Korean guidelines recommend that physicians should be reviewed every 1–2 months or even earlier. This survey showed that most physicians (92.6%, Fig. 5) followed the guidelines well by prescribing opioids at 1–4-week intervals. In the United States, if a patient has a low risk of drug abuse or low dose fluctuations, the patient’s visit interval may be prolonged, and the pros and cons of continuous treatment for 3 months or more will be evaluated [23].
Korean guidelines recommend that patients who need long-term therapy be evaluated every 1–2 months or even earlier. Patients with a high risk of aberrant drug-related behaviors or those requiring frequent dose adjustments should have shorter visit intervals. Aberrant drug-related behaviors, analgesia, adverse effects, and the activities of daily living should be described at every visit [16].
Breakthrough pain should be evaluated separately from baseline pain and may be related to the progression of the underlying condition or a new or unrelated pain condition [41]. The Food and Drug Administration (FDA) has recommended that long-acting opioids not be used on a pro re nata (PRN) or as-needed basis [35]. Canadian guidelines state that most breakthrough pain should be treated with IR opioids at no more than 10%–20% of the total daily dose. If a patient consistently uses breakthrough doses several times a day (three or more times) [16], the continuous dose can be increased using controlled-release opioids. In addition, IR opioids can be administered immediately before severe pain exacerbation [14]. In this survey, most physicians (92.6%) preferred IR opioids to control breakthrough pain. This prescription tendency complied with the FDA and Canadian guidelines, as mentioned earlier. Only 5.9% of patients were prescribed an additional dose of the current medication.
As Korea has restrictions on opioid prescriptions, the NHIS provides a monitoring system for patients on opioids. The NHIS also restricts prescription intervals because of concerns about opioid abuse or addiction, and sometimes this causes insufficient control of pain [16]. We surveyed physicians about the restrictions on prescription intervals, and the results are summarized in Fig. 7. More than half of the physicians (52.9%) thought this restriction was appropriate, while 45.6% responded that the prescription intervals should be increased. Regarding the dose limits covered by the NHIS, 50% of physicians insisted that the dose limit should be increased, while 45.6% thought that the current restriction was appropriate. These results indicate that many physicians thought that some patients needed more opioids for pain control than the dose limit recommended by the NHIS, and some physicians encountered drug tolerance to opioids during long-term opioid prescription. For more sufficient pain control in patients with chronic pain, increasing the dose limit may be necessary. However, the dose limit can be a Maginot line that prevents addicts from being prescribed more drugs covered by insurance. A dose limit may also be a factor that allows physicians to find the best treatment method, such as a combination of drugs, nerve blocks, or other interventions within the limitation. Therefore, to increase the dose limit, much discussion and research are needed in the future.
Even though only 2.9% of physicians thought that the NHIS should decrease the dose limit, the most common reason for decreasing the dose limit was concern about abuse and addiction (67.6%). As mentioned previously, concern about abuse and addiction was the main reason why physicians hesitated to use opioids actively. The most common factors considered when prescribing opioids were the persistence of effectiveness (33.7%), side effects (21.5%), and patient compliance (19.5%). Insurance standards (14.1%), rapid effect (8.3%), and the patients’ financial burden (2%) were minor factors. Therefore, pain physicians had a greater interest in safer and more effective medical treatment than other administrative and financial factors. The side effects of opioids that physicians frequently encounter include constipation, nausea or vomiting, dizziness, dysuria, abuse or addiction, and respiratory depression. Interestingly, the most concerning adverse effects were abuse or addiction, followed by respiratory depression. Both were the least experienced side effects but the most concerning.
Nausea, vomiting, constipation, dizziness, and dysuria were relatively less concerning factors. Constipation is the most common long-term side effect, and opioid-associated constipation generally does not improve with time [6,41]. Respiratory depression is the most critical adverse effect of opioids as it can be life-threatening and typically occurs in individuals taking high doses of opioids [6]. Approximately 80% of patients taking opioids experience at least one side effect [42].
Opioid use disorder refers to the continued use of opioids despite the harmful effects. According to the Diagnostic and Statistical Manual of Mental Disorders Fifth Edition, substance use disorders include abuse and dependence, which are considered not as separate disorders but as a single disorder with symptoms of different severities [43]. As the use of opioids has increased dramatically in many countries, the abuse of prescribed opioids and accidental opioid overdoses have been increasingly observed [44,45]. If a patient’s opioid dose increases frequently or abruptly, incorrect medication or opioid abuse should be suspected despite pain improvement. As the number of patients taking opioids has increased, the opioid overdose-related death toll has also increased [46] and opioid dependence has become a public health crisis in many countries [45,47]. For example, in the United States, northeastern states run the Prescription Drug Monitoring Program to address overprescribing, which results in less opioid prescribing volume in the northeastern states than that in the southern states [48]. The initiative has contributed to the decrease in opioid prescription in the United States since 2013, though the amount of opioids prescribed is still three times higher than in 1999 [49] .
Addiction is a primary neurobiological disease with genetic and psychosocial factors [43] and can be characterized by symptoms such as an inability to control the urge to take the drug and craving [14]. Many patients with opioid addiction also have concomitant psychological disorders [11]. Those with a history of alcohol or other drug addiction seemed to be at the most significant risk [14]. Opioid addiction arises from repeated exposure to opioids [22,50]. As discussed previously, abuse and addiction were the most concerning side effects for physicians. It is worth investigating how many patients have experienced opioid abuse or addiction. As shown in Fig. 12, 56.5% of physicians said that fewer than 5% of patients prescribed opioids misused them, and 66.7% reported that fewer than 5% of patients receiving opioid treatment were addicted.
However, in a survey of Korean patients [38], only 2% of patients were reported to have experienced addiction. Since patients try to avoid revealing opioid addiction by hiding behaviors or symptoms suggesting addiction, diagnosis of addiction can be difficult [14]. Moreover, there are no physical instruments to measure the degree of pain or addiction. The degree of pain can only be reported by patients themselves, and the degree of addiction can only be ambiguously determined [44]. Effective alleviation of suffering associated with pain falls within the physician’s professional obligation. The current situation of opioid prescription should be considered in the amendment of the first Korean guidelines by the Korean Pain Society.
It should be noted that this study is subject to some limitations. Since the survey was conducted only in university hospitals, pain physicians in primary and secondary hospitals were not included; therefore, the study does not reflect all current prescribing trends.
In this study, the prescription patterns of pain physicians generally followed Korean guidelines. All physicians in the survey insisted that they began to prescribe opioids to patients with NRS pain scores > 4. Physicians gave the greatest consideration to safety and effectiveness during prescription, and they were most concerned about respiratory depression and addiction or abuse. The survey revealed the pros and cons of the restrictions on prescription intervals and doses imposed by the NHIS. However, many physicians agreed that regulations needed improvement for patient convenience and safety, and foreffective treatment.
Notes
DATA AVAILABILITY
Data files are available from Harvard Dataverse: https://doi.org/10.7910/DVN/J69VVB.
REFERENCES
1. Nadeau SE. 2015; Opioids for chronic noncancer pain: to prescribe or not to prescribe-what is the question? Neurology. 85:646–51. DOI: 10.1212/WNL.0000000000001766. PMID: 26138946. PMCID: PMC4548284. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84939506338&origin=inward.
2. Hogan ME, Taddio A, Katz J, Shah V, Krahn M. 2017; Health utilities in people with chronic pain using a population-level survey and linked health care administrative data. Pain. 158:408–16. DOI: 10.1097/j.pain.0000000000000776. PMID: 27902568. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85015777826&origin=inward.

3. Fredheim OM, Kaasa S, Fayers P, Saltnes T, Jordhøy M, Borchgrevink PC. 2008; Chronic non-malignant pain patients report as poor health-related quality of life as palliative cancer patients. Acta Anaesthesiol Scand. 52:143–8. DOI: 10.1111/j.1399-6576.2007.01524.x. PMID: 18005378. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=37549051381&origin=inward.

4. Manchikanti L, Singh V, Datta S, Cohen SP, Hirsch JA. American Society of Interventional Pain Physicians. 2009; Comprehensive review of epidemiology, scope, and impact of spinal pain. Pain Physician. 12:E35–70. DOI: 10.36076/ppj.2009/12/E35. PMID: 19668291.
5. Ballantyne JC, LaForge SK. 2007; Opioid dependence and addiction during opioid treatment of chronic pain. Pain. 129:235–55. Erratum in: Pain 2007; 131: 350. DOI: 10.1016/j.pain.2007.07.021. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=34548493466&origin=inward.

6. Warner EA. 2012; Opioids for the treatment of chronic noncancer pain. Am J Med. 125:1155–61. DOI: 10.1016/j.amjmed.2012.04.032. PMID: 22944349. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84869222793&origin=inward.

7. Busse JW, Craigie S, Juurlink DN, Buckley DN, Wang L, Couban RJ, et al. 2017; Guideline for opioid therapy and chronic noncancer pain. CMAJ. 189:E659–66. DOI: 10.1503/cmaj.170363. PMID: 28483845. PMCID: PMC5422149. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85019153936&origin=inward.

8. Kim ED, Lee JY, Son JS, Byeon GJ, Yeo JS, Kim DW, et al. 2017; Guidelines for prescribing opioids for chronic non-cancer pain in Korea. Korean J Pain. 30:18–33. DOI: 10.3344/kjp.2017.30.1.18. PMID: 28119768. PMCID: PMC5256264. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85010471669&origin=inward.

9. Chou R, Qaseem A, Snow V, Casey D, Cross JT Jr, Shekelle P, et al. 2007; Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 147:478–91. Erratum in: Ann Intern Med 2008; 148: 247-8. DOI: 10.7326/0003-4819-147-7-200710020-00006. PMID: 17909209. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=34848925500&origin=inward.

10. Han T. 2001; Opioids in cancer and non-cancer pain management in Korea: the past, present and future. Eur J Pain. 5 Suppl A:73–8. DOI: 10.1053/eujp.2001.0284. PMID: 11798222. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0035696241&origin=inward.

11. Cho NR, Chang YJ, Lee D, Kim JR, Ko DS, Choi JJ. 2021; Trends in opioid prescribing practices in South Korea, 2009-2019: are we safe from an opioid epidemic? PLoS One. 16:e0250972. DOI: 10.1371/journal.pone.0250972. PMID: 33979378. PMCID: PMC8115784. PMID: 69d86f514f0541bfa44b009bf800f6cc. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85105620114&origin=inward.

12. Kim K, Lee H, Shin JY. 2020; Explosive increase in tramadol use in Korea 2003-2013: analysis of patient trends based on the Korea National Health Insurance Database. J Psychoactive Drugs. 52:153–61. DOI: 10.1080/02791072.2019.1612125. PMID: 31079571. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85086052347&origin=inward.

13. Gostin LO, Hodge JG Jr, Noe SA. 2017; Reframing the opioid epidemic as a national emergency. JAMA. 318:1539–40. DOI: 10.1001/jama.2017.13358. PMID: 28832871. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85033236376&origin=inward.

14. Kahan M, Mailis-Gagnon A, Wilson L, Srivastava A. National Opioid Use Guideline Group. 2011; Canadian guideline for safe and effective use of opioids for chronic noncancer pain: clinical summary for family physicians. Part 1: general population. Can Fam Physician. 57:1257–66. e407–18. PMID: 22084455. PMCID: PMC3215602. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=81855218048&origin=inward.
15. Oh TK, Jeon YT, Choi JW. 2019; Trends in chronic opioid use and association with five-year survival in South Korea: a population-based cohort study. Br J Anaesth. 123:655–63. DOI: 10.1016/j.bja.2019.08.012. PMID: 31558315. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85072384311&origin=inward.

16. Baik JS. 2017; Guidelines for prescribing opioids for chronic non-cancer pain in Korea: can you overcome "opiophobia"? Korean J Pain. 30:1–2. DOI: 10.3344/kjp.2017.30.1.1. PMID: 28119766. PMCID: PMC5256267. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85010297602&origin=inward.

17. Kim MH, Park H, Park EC, Park K. 2011; Attitude and knowledge of physicians about cancer pain management: young doctors of South Korea in their early career. Jpn J Clin Oncol. 41:783–91. DOI: 10.1093/jjco/hyr043. PMID: 21502282. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=79957859396&origin=inward.

18. Le Merrer J, Becker JA, Befort K, Kieffer BL. 2009; Reward processing by the opioid system in the brain. Physiol Rev. 89:1379–412. DOI: 10.1152/physrev.00005.2009. PMID: 19789384. PMCID: PMC4482114. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=70349682196&origin=inward.

19. O'Brien T, Christrup LL, Drewes AM, Fallon MT, Kress HG, McQuay HJ, et al. 2017; European Pain Federation position paper on appropriate opioid use in chronic pain management. Eur J Pain. 21:3–19. DOI: 10.1002/ejp.970. PMID: 27991730. PMCID: PMC6680203. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85006314811&origin=inward.
20. Jani M, Birlie Yimer B, Sheppard T, Lunt M, Dixon WG. 2020; Time trends and prescribing patterns of opioid drugs in UK primary care patients with non-cancer pain: a retrospective cohort study. PLoS Med. 17:e1003270. DOI: 10.1371/journal.pmed.1003270. PMID: 33057368. PMCID: PMC7561110. PMID: 3e1558bf693f404889fcdbe2fa9409b0. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85093490305&origin=inward.

21. Barnett ML, Olenski AR, Jena AB. 2017; Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med. 376:663–73. DOI: 10.1056/NEJMsa1610524. PMID: 28199807. PMCID: PMC5428548. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85013498947&origin=inward.

22. Franklin GM. American Academy of Neurology. 2014; Opioids for chronic noncancer pain: a position paper of the American Academy of Neurology. Neurology. 83:1277–84. DOI: 10.1212/WNL.0000000000000839. PMID: 25267983. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84921766370&origin=inward.

23. Dowell D, Haegerich TM, Chou R. 2016; CDC guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. 65:1–49. Erratum in: MMWR Recomm Rep 2016; 65: 295. DOI: 10.15585/mmwr.rr6501e1. PMID: 26987082. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84978823999&origin=inward.

24. Gaskin DJ, Richard P. 2012; The economic costs of pain in the United States. J Pain. 13:715–24. DOI: 10.1016/j.jpain.2012.03.009. PMID: 22607834. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84864445461&origin=inward.

25. Castañeda AM, Lee CS, Kim YC, Lee D, Moon JY. 2018; Addressing opioid-related chemical coping in long-term opioid therapy for chronic noncancer pain: a multicenter, observational, cross-sectional study. J Clin Med. 7:354. DOI: 10.3390/jcm7100354. PMID: 30322212. PMCID: PMC6210168. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85085120941&origin=inward.

26. Boudreau D, Von Korff M, Rutter CM, Saunders K, Ray GT, Sullivan MD, et al. 2009; Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf. 18:1166–75. DOI: 10.1002/pds.1833. PMID: 19718704. PMCID: PMC3280087. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=67651094525&origin=inward.

27. Petzke F, Bock F, Hüppe M, Nothacker M, Norda H, Radbruch L, et al. 2020; Long-term opioid therapy for chronic noncancer pain: second update of the German guidelines. Pain Rep. 5:e840. DOI: 10.1097/PR9.0000000000000840. PMID: 32904018. PMCID: PMC7447355. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85105915220&origin=inward.

28. Chou R, Huffman LH. American Pain Society. American College of Physicians. 2007; Medications for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 147:505–14. Erratum in: Ann Intern Med 2008; 148: 247-8. DOI: 10.7326/0003-4819-147-7-200710020-00008. PMID: 17909211. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=34848826292&origin=inward.

29. Eisenberg E, McNicol ED, Carr DB. 2005; Efficacy and safety of opioid agonists in the treatment of neuropathic pain of nonmalignant origin: systematic review and meta-analysis of randomized controlled trials. JAMA. 293:3043–52. DOI: 10.1001/jama.293.24.3043. PMID: 15972567. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=20644431597&origin=inward.

30. Barbera L, Seow H, Husain A, Howell D, Atzema C, Sutradhar R, et al. 2012; Opioid prescription after pain assessment: a population-based cohort of elderly patients with cancer. J Clin Oncol. 30:1095–9. DOI: 10.1200/JCO.2011.37.3068. PMID: 22370317. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84863951751&origin=inward.

31. Beloeil H, Albaladejo P, Sion A, Durand M, Martinez V, Lasocki S, et al. 2019; Multicentre, prospective, double-blind, randomised controlled clinical trial comparing different non-opioid analgesic combinations with morphine for postoperative analgesia: the OCTOPUS study. Br J Anaesth. 122:e98–106. DOI: 10.1016/j.bja.2018.10.058. PMID: 30915987. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85059165166&origin=inward.

32. Carville SF, Arendt-Nielsen L, Bliddal H, Blotman F, Branco JC, Buskila D, et al. 2008; EULAR evidence-based recommendations for the management of fibromyalgia syndrome. Ann Rheum Dis. 67:536–41. Erratum in: Ann Rheum Dis 2015; 74: 476. DOI: 10.1136/ard.2007.071522. PMID: 17644548. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=41849093775&origin=inward.

33. Lee J, Lim YH, Hong SJ, Jeong JH, Choi HR, Park SK, et al. 2021; Multicenter survey of symptoms, work life, economic status, and quality of life of complex regional pain syndrome patients. Korean J Pain. 34:288–303. DOI: 10.3344/kjp.2021.34.3.288. PMID: 34193635. PMCID: PMC8255153. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85110343207&origin=inward.

34. Busse JW, Wang L, Kamaleldin M, Craigie S, Riva JJ, Montoya L, et al. 2018; Opioids for chronic noncancer pain: a systematic review and meta-analysis. JAMA. 320:2448–60. DOI: 10.1001/jama.2018.18472. PMID: 30561481. PMCID: PMC6583638. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85058673821&origin=inward.
35. Manchikanti L, Kaye AM, Knezevic NN, McAnally H, Slavin K, Trescot AM, et al. 2017; Responsible, safe, and effective prescription of opioids for chronic non-cancer pain: American Society of Interventional Pain Physicians (ASIPP) guidelines. Pain Physician. 20(2S):S3–92. DOI: 10.36076/ppj.2017.s92. PMID: 28226332.
36. Woo A, Lechner B, Fu T, Wong CS, Chiu N, Lam H, et al. 2015; Cut points for mild, moderate, and severe pain among cancer and non-cancer patients: a literature review. Ann Palliat Med. 4:176–83. DOI: 10.3978/j.issn.2224-5820.2015.09.04. PMID: 26541396. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84987625579&origin=inward.
37. Kim J, Shin S, Jeong Y, Kim SY, Lee HJ. 2020; Medicolegal consideration to prevent medical malpractice regarding opioid administration: an analysis of judicial opinion in South Korea. J Pain Res. 13:1525–32. DOI: 10.2147/JPR.S256759. PMID: 32612380. PMCID: PMC7323961. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85086874080&origin=inward.
38. Kim CL, Hong SJ, Lim YH, Jeong JH, Moon HS, Choi HR, et al. 2020; Patients' perception about opioids and addiction in South Korea. Korean J Pain. 33:234–44. DOI: 10.3344/kjp.2020.33.3.234. PMID: 32606268. PMCID: PMC7336346. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85091747747&origin=inward.

39. Hanks GW, Conno F, Cherny N, Hanna M, Kalso E, McQuay HJ, et al. 2001; Morphine and alternative opioids in cancer pain: the EAPC recommendations. Br J Cancer. 84:587–93. DOI: 10.1054/bjoc.2001.1680. PMID: 11237376. PMCID: PMC2363790. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0037758126&origin=inward.

40. Smith HS. 2008; Combination opioid analgesics. Pain Physician. 11:201–14. DOI: 10.36076/ppj.2008/11/201. PMID: 18354712.

41. Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P, et al. 2009; Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 10:113–30. DOI: 10.1016/j.jpain.2008.10.008. PMID: 19187889. PMCID: PMC4043401. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=58949087497&origin=inward.

42. Kalso E, Edwards JE, Moore AR, McQuay HJ. 2004; Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain. 112:372–80. DOI: 10.1016/j.pain.2004.09.019. PMID: 15561393.

43. Kaye AD, Jones MR, Kaye AM, Ripoll JG, Galan V, Beakley BD, et al. 2017; Prescription opioid abuse in chronic pain: an updated review of opioid abuse predictors and strategies to curb opioid abuse: part 1. Pain Physician. 20(2S):S93–109. DOI: 10.36076/ppj.2017.s109. PMID: 28226333.
44. Salsitz EA. 2016; Chronic pain, chronic opioid addiction: a complex nexus. J Med Toxicol. 12:54–7. DOI: 10.1007/s13181-015-0521-9. PMID: 26602212. PMCID: PMC4781803. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84948132277&origin=inward.

45. Soffin EM, Lee BH, Kumar KK, Wu CL. 2019; The prescription opioid crisis: role of the anaesthesiologist in reducing opioid use and misuse. Br J Anaesth. 122:e198–208. DOI: 10.1016/j.bja.2018.11.019. PMID: 30915988. PMCID: PMC8176648. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85059153949&origin=inward.

46. Webster LR. 2017; Risk factors for opioid-use disorder and overdose. Anesth Analg. 125:1741–8. DOI: 10.1213/ANE.0000000000002496. PMID: 29049118. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85032450877&origin=inward.

47. Clark DJ, Schumacher MA. 2017; America's opioid epidemic: supply and demand considerations. Anesth Analg. 125:1667–74. DOI: 10.1213/ANE.0000000000002388. PMID: 29049112. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85032440107&origin=inward.
48. Champagne-Langabeer T, Madu R, Giri S, Stotts AL, Langabeer JR. 2021; Opioid prescribing patterns and overdose deaths in Texas. Subst Abus. 42:161–7. DOI: 10.1080/08897077.2019.1675114. PMID: 31644388. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85074584595&origin=inward.

49. Guy GP Jr, Zhang K, Bohm MK, Losby J, Lewis B, Young R, et al. 2017; Vital signs: changes in opioid prescribing in the United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 66:697–704. DOI: 10.15585/mmwr.mm6626a4. PMID: 28683056. PMCID: PMC5726238. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85024105633&origin=inward.

50. Kolodny A, Courtwright DT, Hwang CS, Kreiner P, Eadie JL, Clark TW, et al. 2015; The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health. 36:559–74. DOI: 10.1146/annurev-publhealth-031914-122957. PMID: 25581144. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84925260632&origin=inward.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download