INTRODUCTION
Currently available drugs to treat inflammatory pain include corticosteroids and non-steroidal anti-inflammatory drugs (NSAIDs) [
1]. The mechanism of action of NSAIDs is mainly the inhibition of cyclooxygenase (COX), which is the key enzyme in the conversion of arachidonic acid to prostaglandins [
2].
In 1991, it was discovered that in mammals the two isoforms of this enzyme, COX-1 and COX-2, have independent genes and different expression patterns [
3]. Following this classification, extensive research conducted for the synthesis of selective COX-2 inhibitors and several drugs including celecoxib and rofecoxib were marketed [
4,
5]. Compared to traditional NSAIDs, these drugs had fewer gastrointestinal side effects [
6–
8], but after a short time, it was reported that they caused cardiovascular side effects such as thrombosis, myocardial infarction, and cardiac dysfunction [
9–
13]. The proposed mechanism for these effects is the inhibition of prostacyclin production in vessels which mediate platelet activation and atherogenesis [
14]. Except celecoxib, other selective COX-2 inhibitors were withdrawn from the market due to these cardiovascular complications [
15]. Therefore, the search for novel drugs devoid of the mentioned side effects is still an ongoing need for treatment of pain and inflammation.
Heterocyclic compounds have played an important role in the structure of anti-inflammatory drugs. Numerous synthesis methods have been developed to improve the NSAID structures through chemical modification of heterocyclic rings. Celecoxib and the other coxibs are diaryl heterocycles [
16]. Studies on the structure-activity relationship of diaryl heterocycles have shown that the presence of a group of SO2NH2 or SO2Me in the para position of one of the aryl rings often provides the optimum activity for activity and specificity against COX-2 [
17]. Compounds in which the pyrazole ring has been substituted with the pyrazoline, oxazolone, oxadiazolone, maleimide, and even quinazoline alternatives have also shown potent and specific COX-2 inhibition [
18]. In a large number of biologically active heterocyclic compounds, the triazine ring plays an important role in the observed activity. Triazine derivatives in the form of 1, 2, 4-triazine have also shown analgesic activity [
19,
20].
Considering the structural properties of COX-2 inhibitors, Asadi et al. [
21] synthesized new compounds with a heterocyclic ring based on triazine and evaluated their anti-inflammatory effect. In the continuation of this research, the present study aimed to evaluate the antinociceptive effects of selected triazine derivatives in mice.
DISCUSSION
In the authors’ previous work, five out of nine novel triazine compounds were found to have potent anti-inflammatory activity in a carrageenan-induced paw edema model in rats [
21].
In this study, these five compounds including two vanillin-triazine and three phenylpyrazole-triazine derivatives, which were evaluated for their possible antinociceptive activity in animal models and all of them showed a significant antinociceptive effect. These findings are in agreement with previous reports on the analgesic activity of some other triazine derivatives [
19,
20]. In the present study three different animal models, including acetic acid-induced writhing, formalin, and hot plate tests were used to evaluate the antinociceptive effect of the triazine compounds.
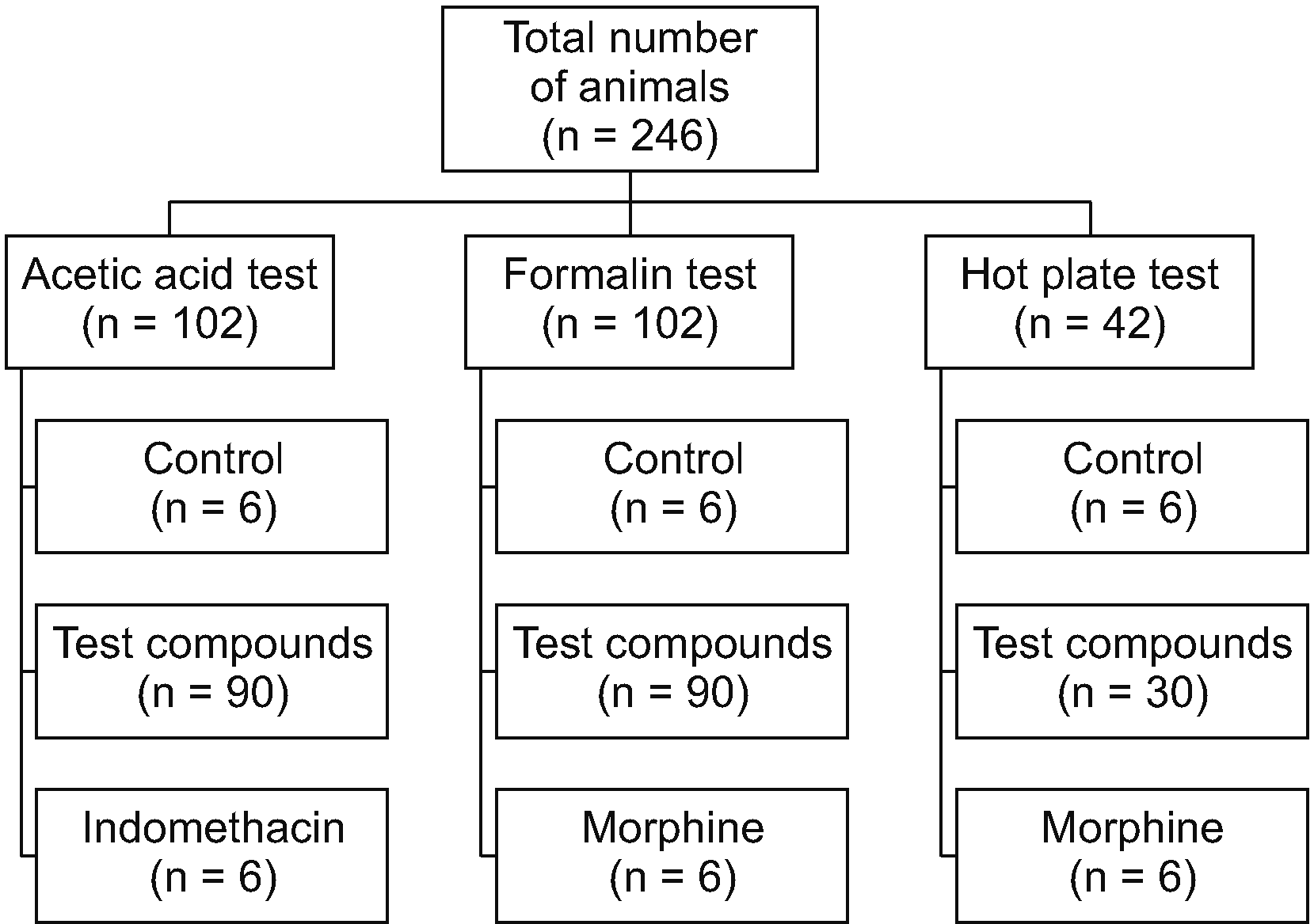
The acetic acid-induced writhing test has been widely used as a screening test for assessing pain killers or anti-inflammatory drugs. Following injection of acetic acid into the peritoneal cavity of mice, the animals show a stretching behavior also called writhing. This chemical agent induces both pain and inflammation by the stimulation of nociceptors and release of inflammatory mediators, respectively. Acetic acid test is considered a non-selective model because NSAIDs, opioids, calcium channel blockers, antispasmodic drugs, and some other drugs are able to reduce the frequency of writhing and show antinociceptive effect in this model. Despite the non-selectivity of the test, it has been known as the most frequently used screening test for antinociceptive compounds [
22,
26].
In the acetic acid test, all five triazine derivatives significantly reduced the number of writhings and, as expected, indomethacin as the reference drug also significantly controlled the pain behavior. The percentage of the inhibition of abdominal contractions showed that at the maximum tested dose of these compounds (200 mg/kg), the order of their potencies is as follows:
10b > 10a > 10e > 5d > 5c. Indomethacin at a dose of 10 mg/kg exerted 95% inhibition of the acetic acid-induced writhes and the effect of the 10b, 10a, and 10e compounds were comparable with that of indomethicin.
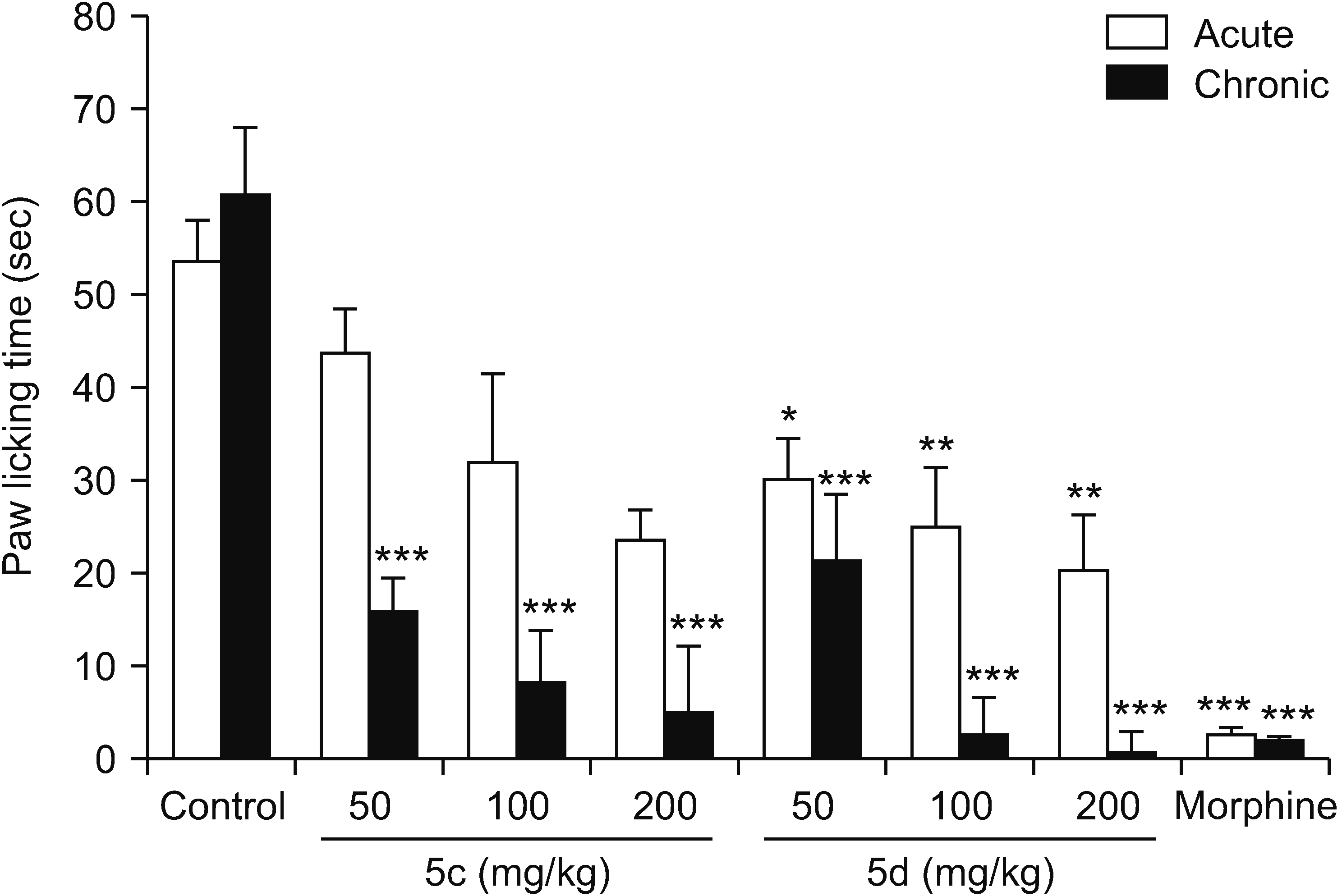
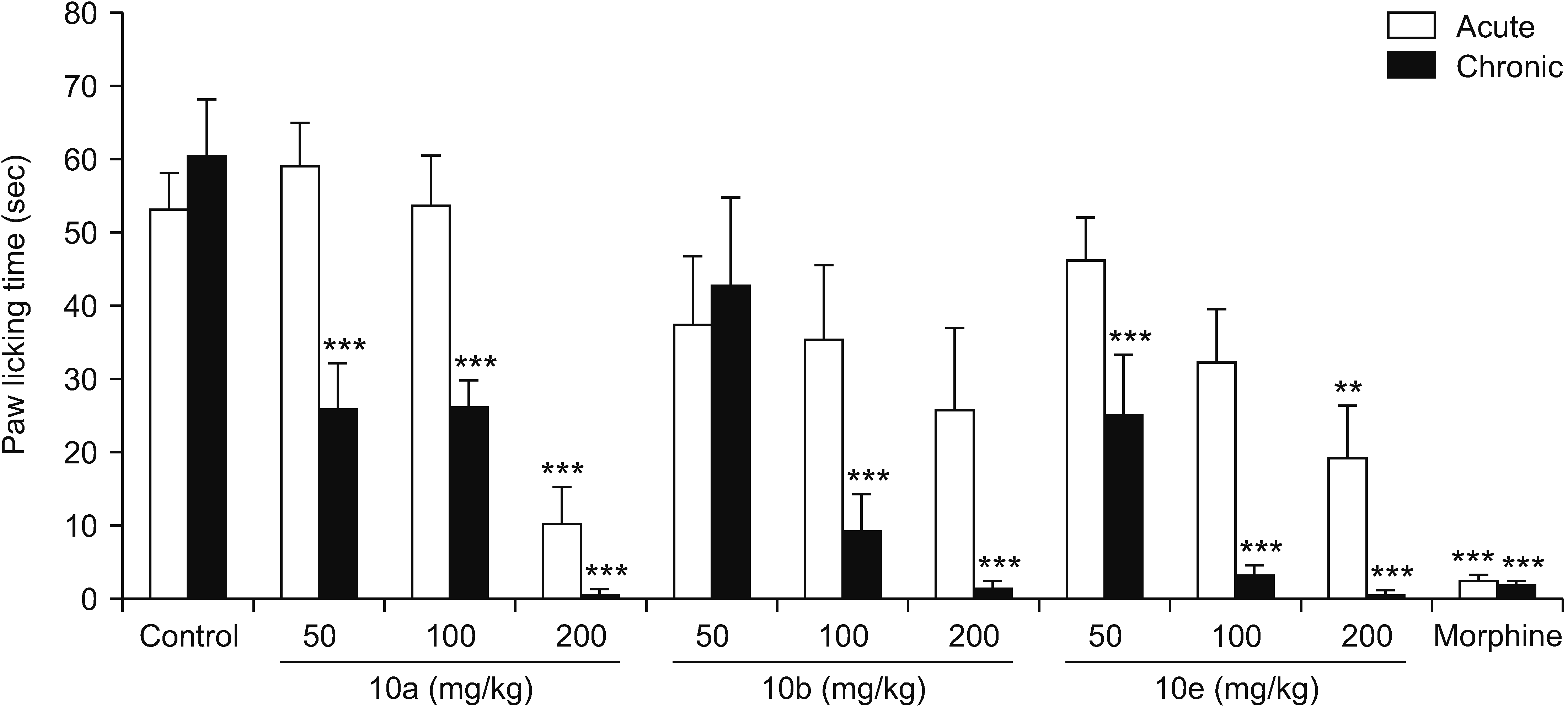
In the formalin test, this study’s results clearly showed that an acute 2.5% formalin injection produced two phases of the nociceptive process in mice. The formalin test is known as a useful model for screening novel compounds because it simultaneously reveals the inflammatory, neurogenic, and central mechanisms of pain behavior. Intraplantar injection of formalin into the hind paw produced a typical pain characterized by two phases. In the early phase which began just after the formalin injection, the licking of the injected paw was recorded for 5 minutes and in the second phase, also called the late phase or chronic phase, the licking behavior was recorded in a 20 minutes period which started 20 minutes after formalin administration.
According to previous reports, prostaglandins do not play an important role during the early phase and therefor NSAIDs as inhibitors of prostaglandin synthesis could not suppress this phase [
23,
27,
28]. Based on this study’s results, compound 5d at all the tested doses (50, 100, and 200 mg/kg) and compounds 10a and 10e at a dose of 200 mg/kg significantly inhibited the early phase, and this means that these compounds might have additional mechanisms of action. Also, the molecular docking performed in the previous study predicted that the best compounds for inhibition of COX-2 were 5c and 10c while, surprisingly, the pharmacological study showed that compounds 5c and 5d among the vanillin derivatives and 10a, 10b, and 10e among the phenylpyrazole-triazine derivatives had the best anti-inflammatory activity [
21], and the present study also emphasized the potent antinociceptive effect of these compounds. Therefore, it might be concluded that mechanisms other than inhibition of COX-2 might also be involved, but further studies are needed for determining a definite mechanism. Consistent with this viewpoint, Choi et al. [
29] reported that DUP-697 as a selective COX-2 inhibitor reduced the pain response evoked by formalin injection during both phases and, using some opioid receptor antagonists, they concluded that the endogenous opioid system is involved in its antinociceptive effect.
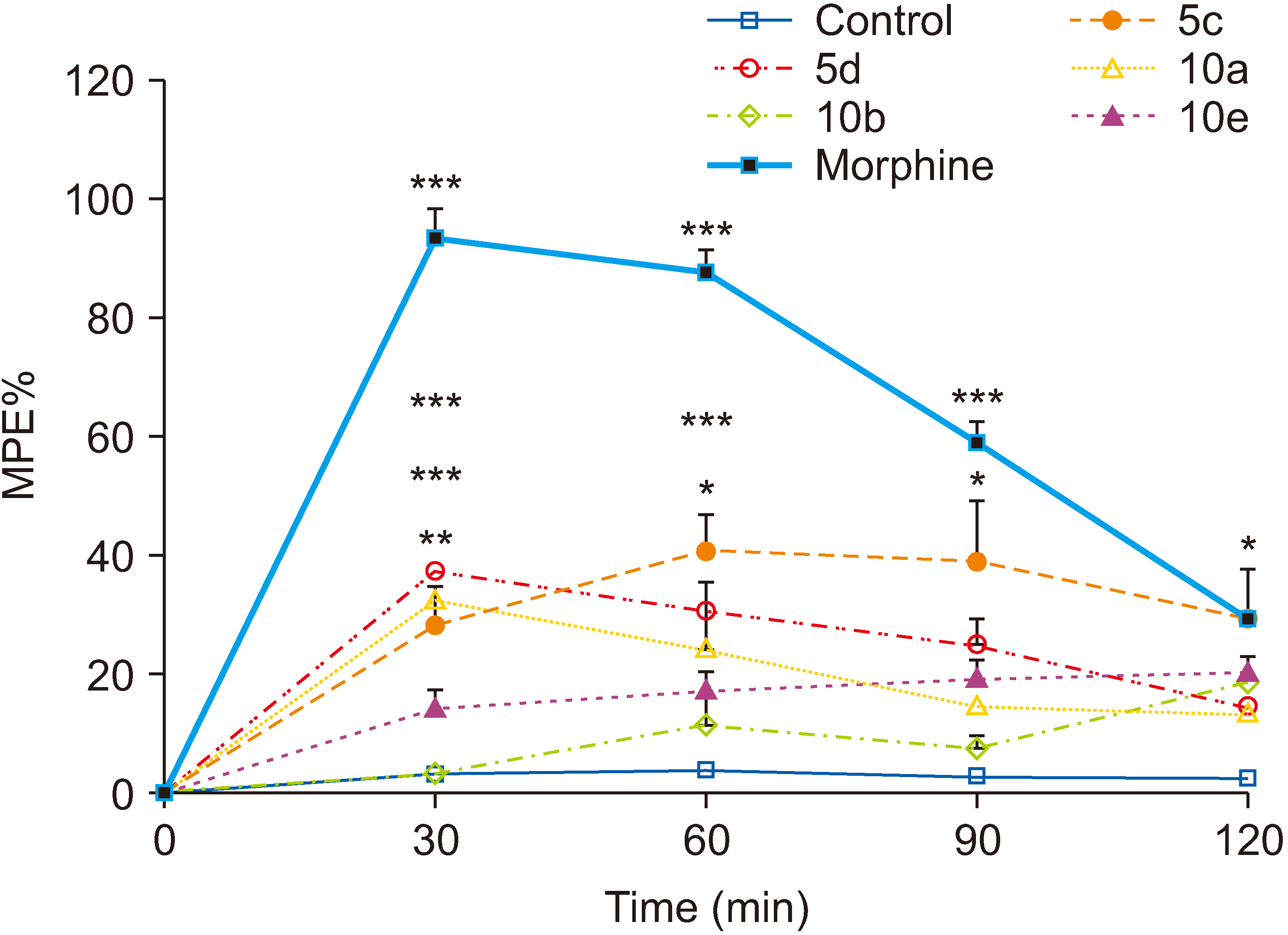
Compounds 5c, 5d, and 10a also showed significant antinociceptive effect in the hot plate test. The hot plate test has an advantage over other thermal models in that repeated placement of the same animal on the plate and at different time intervals over a short period of time (2–3 hours) does not cause tissue injury [
30,
31]. In this test, foot-licking and jumping are the two parameters commonly measured as indexes of pain severity. Analgesics increase the latency to licking/jumping. NSAIDs like aspirin and ibuprofen, and also paracetamol, demonstrate little antinociceptive effect in this test while centrally-acting drugs like opioids show potent effect and therefor the hot plate model has been considered a very useful method to detect centrally-acting analgesics [
29–
32]. According to the authors’ findings that compounds 5c, 5d, and 10a showed significant antinociceptive effect in the hot plate model, it might be concluded that in addition to peripheral activity which was confirmed in the acetic acid test and in the chronic phase of the formalin test, central mechanisms are also involved.
In conclusion, the results clearly showed that both vanillin-triazine and phenylpyrazole-triazine derivatives had an antinociceptive effect. Also, some compounds which showed activity in the early phase of the formalin test as well as in the hot plate test could control acute pain in addition to chronic or inflammatory pain.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download