Abstract
Background
Repeated administration of opioid analgesics for pain treatment can produce paradoxical hyperalgesia via peripheral and/or central mechanisms. Thus, this study investigated whether spinally (centrally) administered orexin A attenuates opioid-induced hyperalgesia (OIH).
Methods
[D-Ala2, N-Me-Phe4, Gly5-ol]-enkephalin (DAMGO), a selective µ-opioid receptor agonist, was used to induce mechanical hypersensitivity and was administered intradermally (4 times, 1-hour intervals) on the rat hind paw dorsum. To determine whether post- or pretreatments with spinal orexin A, dynorphin A, and anti-dynorphin A were effective in OIH, the drugs were injected through an intrathecal catheter whose tip was positioned dorsally at the L3 segment of the spinal cord (5 µg for all). Mechanical hypersensitivity was assessed using von Frey monofilaments.
Results
Repeated intradermal injections of DAMGO resulted in mechanical hypersensitivity in rats, lasting more than 8 days. Although the first intrathecal treatment of orexin A on the 6th day after DAMGO exposure did not show any significant effect on the mechanical threshold, the second (on the 8th day) significantly attenuated the DAMGO-induced mechanical hypersensitivity, which disappeared when the type 1 orexin receptor (OX1R) was blocked. However, intrathecal administration of dynorphin or an anti-dynorphin antibody (dynorphin antagonists) had no effect on DAMGO-induced hypersensitivity. Lastly, pretreatment with orexin A, dynorphin, or anti-dynorphin did not prevent DAMGO-induced mechanical hypersensitivity.
Opioid analgesics are used to effectively treat chronic pain. However, repeated administration of opioids can induce paradoxical progressive hyperalgesia, a phenomenon referred to as opioid-induced hyperalgesia (OIH) [1]. This type of hyperalgesia is currently a key obstacle, with opioid tolerance and dependence, in the use of opioids for the treatment of chronic pain. Despite accumulating evidence, the underlying mechanisms of OIH are still not fully understood [2,3]. However, among the mechanisms proposed to date, the involvement of spinal glutamate is suggested to be especially important [2]. The initial downregulation of glutamate transporters by chronic morphine administration increased the level of glutamate in the spinal dorsal horn due to the decrease in glutamate uptake [4]. This increased glutamate level induces neurotoxicity via activation of the NMDA receptor, an ionotropic glutamate receptor [4], potentially decreasing the number of inhibitory GABAergic neurons in the spinal dorsal horn [5]. Another suggested mechanism is that continuous infusions of µ-opioid receptor (MOR) agonists increase spinal levels of dynorphin [6], causing spinal release of pro-nociceptive neuropeptides such as calcitonin gene-related peptide (CGRP) from peptidergic primary afferent fibers [7]. Both mechanisms of OIH introduced here are related to sensitizing changes in spinal dorsal horn neurons.
Although the neuropeptides orexins are known as regulators of wakefulness and feeding behaviors [8–10], there has been some indication that orexins have an ability to modulate spinal neurotransmission and neuronal excitability. Particularly, orexin A—a type of orexin—decreases excitatory synaptic transmission and neuronal excitability in the spinal dorsal horn [11]. Additionally, the maintenance of long-term depression—a long-lasting decrease in excitatory synaptic strength involving the potential alleviation of pain hypersensitivity [12]—requires the activation of orexin receptors OX1 and OX2 [13]. According to these electrophysiological data, orexins have been shown to have analgesic effects on neuropathic pain induced by diabetic neuropathy or experimental peripheral nerve ligation [14,15]. Contrastingly, orexinergic neurons, whose cell bodies are located in the hypothalamus and axon fibers are terminated in the spinal dorsal horn, contain dynorphin [16].
In this study, we investigated whether the spinal action of orexin A can alleviate or prevent abnormal pain hypersensitivity, mimicking OIH, which is induced and maintained by repeated intradermal injections of [D-Ala2, N-Me-Phe4, Gly5-ol]-enkephalin (DAMGO) in rats [17]. The involvement of spinal dynorphin is tested to determine whether an increase or decrease in spinal dynorphin levels can affect DAMGO-induced hypersensitivity.
All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Keimyung University Dongsan Hospital (approval number: KM-2019-26R2). Sprague-Dawley rats (male, 200–300 g; Hana Trading Company, Busan, Korea) were placed in a room (24°C –26°C) with an artificial 12-hour day-night light cycle and water and food provided ad libitum. The number of animals and their suffering were minimized. The rats were randomized into each group. Drugs injected on the dorsum of the hind paw of the rat or through an intrathecal catheter were blinded to an experimenter which tested mechanical hypersensitivity with von Frey filaments.
Under isoflurane anesthesia (2.5% isoflurane with 2 L/min 100% O2), a polyethylene catheter (PE-10) was surgically inserted, through the atlanto-occipital membrane, in the lumbar (L) subarachnoid space of each rat, and the tip of the catheter was dorsally positioned around the L1-3 spinal segment [18]. After the surgery, the rats were allowed to recover for 1 week. During the one week-recovery period, the rats, which revealed limitation of paw movement or mechanical hypersensitivity, were excluded.
All rats with an intrathecal catheter were habituated for 2–3 days before the test day. On the test day, a rat was placed on the elevated wire grid and, after 1 hour of additional habituation, the mechanical threshold was measured as the force (g) of the lowest von Frey monofilament that evoked a brisk paw withdrawal response (the initial and maximal monofilament forces of 0.4 g and 30 g, respectively; the force of 30 g was assigned when the subjected rat responding negatively at the application of the 26 g-monofilament). The tip of the von Frey monofilament was applied to the middle portion of the plantar surface of the hind paws separately until the filament bent for approximately 2 seconds. Two withdrawal responses from five trials was considered positive [5,19].
DAMGO, a selective MOR agonist, was used to induce mechanical hypersensitivity [17]. DAMGO solution (solvent: normal saline containing 0.9% NaCl) was administered intradermally on the dorsum of the rat hind paw a total of four times (1-hour intervals; 1 µg/µL; volume, 5 µL each), using a 30-gauge hypodermic needle adapted to a 50 µL Hamilton syringe. Mechanical thresholds were measured 30 minutes before and 1 hour after the 4th administration, and then at the 4th, 6th, or 8th days after DAMGO exposure. In a prior study, standard deviation of the von Frey threshold was 7.56 g on the 6th day, and the effect size of the von Frey test was 13. Therefore, it is estimated that at least 6 rats per group of the sample size are required for proper comparisons in this experiment [20].
Orexin A, SB674042, and dynorphin A were purchased from Tocris Bioscience (Bristol, UK), and the anti-dynorphin A antibody was purchased from Abcam (Cambridge, UK). SB674042 was dissolved in dimethyl sulfoxide (DMSO) and the final solution contained 6% DMSO. The remaining samples were dissolved in saline solution. The amount of intrathecally administered drug was 5 µg in a volume of 5 µL and all PE-10 tubes were flushed with 10 µL of saline [21–24]. All drugs were injected 30 minutes before the von Frey tests.
Data are expressed as the threshold (mean ± SEM; n, number of rats) measured by the mechanical force (g) of the von Frey filaments. To compare the mechanical thresholds after repeated intradermal injections of DAMGO to the baseline, one- or two-way repeated-measures ANOVA was performed. The Wilcoxon signed-rank test was used to identify the effects of intrathecal administration. Differences were considered statistically significant at P < 0.05.
A total of 104 rats were used in this experiment. For catheter insertion, 91 rats were subjected to the surgery, and 10 rats (out of 91) were excluded for further investigation because of neurological signs, such as paw movement limitation, after intrathecal catheter insertion. Others were randomly allocated to each group.
A previous study showed that a form of OIH could be induced by repeated intradermal injection of DAMGO [17], we used the same protocol to induce a mechanical hypersensitivity by repetitively activating peripheral MORs. For evaluation of mechanical allodynia, two groups were investigated. A total of thirteen rats were used for behavior tests after injection. DAMGO solution (concentration, 1 µg/µL; volume, 5 µL, n = 7) was injected intradermally into the dorsum of the rat hind paws a total of four times at 1 hour-intervals. One hour after the final injection the mechanical threshold, measured on the plantar surface of the hind paw, decreased significantly compared to the control saline group (n = 6) (P < 0.01; Fig. 1), indicating an induced mechanical hypersensitivity in the hind paw after repeated DAMGO exposure. DAMGO-induced mechanical hypersensitivity lasted at least a week, since the decreased mechanical threshold was observed in the same hind paw at both 4 and 6 days after the repeated DAMGO injections (P < 0.01 vs. saline group; P < 0.05 or 0.01 vs. baseline threshold before the DAMGO exposure; Fig. 1).
It has been suggested that OIH is accompanied by sensitization of spinal neurons [2]. Contrastingly, we previously observed that orexin A diminishes excitatory transmission in the dorsal horn of the spinal cord [11] and mediates long-term depression of excitatory synaptic transmission via OX1, a type 1 orexin receptor [13]. Therefore, we intrathecally applied orexin A to determine whether its action on excitatory synaptic transmission in the spinal dorsal horn alleviated DAMGO-induced hypersensitivity. In this experiment, two rats were excluded due to limitation of hind paw movement after intrathecal catheter insertion. Other rats were divided into five groups for injections of saline (n = 6), orexin A (n = 7), 6% DMSO (n = 6), orexin A with SB674042, an OX1 antagonist (n = 6), and SB674042 alone (n = 6). After confirmation of mechanical hypersensitivity on the 4th day after repeated intradermal exposure to DAMGO, orexin A (concentration, 1 µg/µL; volume, 5 µL) was administered through a pre-inserted intrathecal catheter positioned dorsally at the L3 level of the spinal cord. Although the first intrathecal treatment of orexin A on the 6th day following repeated DAMGO exposure did not show any significant effect on the mechanical threshold, the second treatment on the 8th day resulted in a significant recovery of the DAMGO-induced mechanical hypersensitivity, whereas the intrathecal administration of saline and 6% DMSO did not affect the mechanical hypersensitivity developed by DAMGO exposure (Fig. 2; P < 0.01 vs. intrathecal saline or 6% DMSO groups). Furthermore, recovery by intrathecal orexin A administration from DAMGO-induced mechanical hypersensitivity was prevented by co-administration of orexin A with SB674042, an OX1 antagonist (Fig. 2).
Contrastingly, OIH increases spinal dynorphin levels, further contributing to the pronociceptive process in the spinal cord [2]. To investigate the potential role of dynorphin in DAMGO-induced hypersensitivity, the authors intrathecally administered either dynorphin or anti-dynorphin antibodies after the development of mechanical hypersensitivity. In this experiment, five rats were excluded due to limitation of paw movement after intrathecal catheter insertion. Three treatment groups were allocated for the effects of dynorphin, anti-dynorphin, and saline (6 rats in each group). Although the 2nd intrathecal administration of dynorphin (5 µg/5 µL) slightly decreased the mechanical hypersensitivity (P > 0.05, compared to before DAMGO injection; Fig. 3) overall, dynorphin or anti-dynorphin antibodies had no effect on the DAMGO-induced mechanical hypersensitivity (Fig. 3).
Furthermore, we investigated whether spinal orexin A, and dynorphin and anti-dynorphin antibodies prevented the development of DAMGO-induced mechanical hypersensitivity. Three rats were excluded due to limitation of hind paw movement. Each group included eight rats for the testing. For these preemptive effects, orexin A, and dynorphin and anti-dynorphin antibodies were intrathecally administered before intradermal injection of DAMGO at a concentration of 1 µg/1 µL (volume, 5 µL). As shown in Fig. 4, none of the pretreatments had an effect on the DAMGO-induced hypersensitivity.
In this study, it was shown that repetitive intradermal injection of DAMGO, a MOR agonist, induced mechanical hypersensitivity in rats that lasted for at least eight days. The induced hypersensitivity was normalized by intrathecally administered orexin A, on the 8th day after induction, but not on the 6th day after induction. The relief of hypersensitivity by orexin A is mediated by its type 1 receptor, OX1. However, neither dynorphin supplementation nor scavenging affected the mechanical DAMGO-induced hypersensitivity. Moreover, the induction of mechanical hypersensitivity was neither prevented by the spinal pretreatments of orexin A or dynorphin nor dependent of the spinal action of dynorphin. Despite the lack of understanding of the underlying mechanism, our results suggest that spinal (i.e., central) orexin A can reverse the mechanical hypersensitivity induced by repetitive peripheral stimulation of the MOR.
A previous study demonstrated that repetitive intradermal injection of DAMGO produced mechanical hypersensitivity, which lasted for at least 4 hours but was not observed after one week [17]. However, an intradermal injection of prostaglandin E2 at the same site injected with DAMGO caused the reappearance of mechanical hypersensitivity after more than one week, a phenomenon called “type II hyperalgesic priming” [25]. This study postulated the occurrence of neuroplastic changes in peripheral nociceptors following repeated DAMGO exposure [17]. Contrastingly, our present study observed mechanical hypersensitivity that lasted for more than a week following repeated DAMGO exposure (up to 8 days). This difference may be due to the test method, as we used an ascending stimulus method utilizing von Frey monofilament [5] whereas the other study used the Randall–Selitto test [17]. Both methods measure mechanical thresholds but would differ in the type or number of activated nociceptive/non-nociceptive fibers [19]; thus, the ascending stimulus method used in this study may be able to detect mechanical hypersensitivity several days after repeated DAMGO exposure.
In this study, DAMGO-induced hypersensitivity due to repeated exposure was normalized, but not prevented, by spinal activation of the orexin A-OX1 receptor pathway. Neurons of the lateral hypothalamus and perifornical area produce the neuropeptide orexin A [26,27], and their axons project to various regions of the brain, including the spinal dorsal horn [28,29], while the OX1 receptor—which has a high affinity for orexin A—is expressed in the gray matter of the spinal cord [30,31] and dorsal root ganglion (DRG) [31]. Orexin A alters nociceptive modulation by inhibiting Ca2+ influx through L-type Ca2+ channels in the DRG [32]. Furthermore, activation of the orexin A-OX1 receptor pathway reduces A/C fiber-mediated excitatory synaptic transmission and contributes to the induction of long-term depression in the spinal dorsal horn [11,13]. Therefore, it is conceivable that the orexin A-OX1 receptor pathway modulates the spinal transmission of pain signals generated by peripheral repetitive activation of the MOR. Particularly, it would be far more effective if the activation of the orexin A-OX1 receptor pathway modulated TRPV1-positive C fiber-mediated synaptic transmission, because DAMGO induces late-onset long-term potentiation in laminae I and II neurons that resemble OIH [33]. However, it should be mentioned that orexin A also has an analgesic effect on other types of persistent pain. Notably, intrathecally administered orexin A has been reported to have an analgesic effect in the hot plate test and reduces the number of laminae I and II neurons with Fos-like immunoreactivity in the spinal dorsal horn [8]. Besides the potential analgesic mechanisms of orexin A mentioned above, a study showed that persistent pain and a certain stressful condition activate orexinergic neurons and inhibit pain transmission [34]. Furthermore, it seems that, in stressful conditions, orexin expression is increased and leads to elevation of animal performance and inhibition of nociceptive signals [35]. Thus, the effect of the intrathecal administration of orexin A would be additive to the effect by the orexin A-mediated analgesic mechanism activated by repeated exposure to DAMGO. This hypothesis needs to be further tested.
The mechanism underlying persistent mechanical hypersensitivity caused by repeated intradermal exposure to DAMGO in this study is unclear. One suggested mechanism is the downregulation of glutamate transporters and activation of NMDA receptors in the spinal dorsal horn [4]. Recently, another possible mechanism that has gained attention is the increased spinal level of dynorphin because it causes spinal release of CGRP [7] and activates spinal bradykinin receptors [36]. Likewise, in neuropathic pain models such as L5/L6 spinal nerve ligation or chronic constriction injury of the sciatic nerve, dynorphin is elevated in the cerebrospinal fluid [21,37]. Consistent with these neurochemical studies, hyperalgesia after intrathecal morphine infusion was reversed by dynorphin A antiserum [38]. Contrastingly, our results revealed a little analgesic effect on DAMGO-induced hypersensitivity since neither the intrathecal dynorphin nor anti-dynorphin antibody reversed or prevented the resulting hypersensitivity. This is similar to a previous study that reported that intrathecal orexin A antiserum itself had no effect in both the formalin and hot plate tests but did abolish the analgesic effect of orexin A on these tests [39].
In conclusion, this study revealed that spinal orexin A reverses peripheral DAMGO-induced mechanical hypersensitivity; however, this treatment does not prevent its induction. Although other studies have suggested prominent roles of spinal dynorphin in OIH, our study did not find any role of dynorphin A in the spinal dorsal horn in the DAMGO-induced hypersensitivity. Overall, although this study does not provide mechanisms for the orexin A’s effectiveness on the DAMGO-induced hypersensitivity in the rats, it suggests in the first time that the OIH, which limits the use of opioids in the treatment of chronic pain, can be treated by spinal administration of orexin A or OX1 agonists.
Notes
DATA AVAILABILITY
Data files are available from Harvard Dataverse: https://doi.org/10.7910/DVN/CYOOXJ.
REFERENCES
1. Kim SH, Stoicea N, Soghomonyan S, Bergese SD. 2014; Intraoperative use of remifentanil and opioid induced hyperalgesia/acute opioid tolerance: systematic review. Front Pharmacol. 5:108. DOI: 10.3389/fphar.2014.00108. PMID: 24847273. PMCID: PMC4021143. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84904654274&origin=inward.

2. Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. 2011; A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 14:145–61. DOI: 10.36076/ppj.2011/14/145. PMID: 21412369.
3. Mao J. 2002; Opioid-induced abnormal pain sensitivity: implications in clinical opioid therapy. Pain. 100:213–7. DOI: 10.1016/S0304-3959(02)00422-0. PMID: 12467992. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0036898884&origin=inward.

4. Mao J, Sung B, Ji RR, Lim G. 2002; Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. J Neurosci. 22:8312–23. DOI: 10.1523/JNEUROSCI.22-18-08312.2002. PMID: 12223586. PMCID: PMC6758088. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0037107189&origin=inward.

5. Scholz J, Broom DC, Youn DH, Mills CD, Kohno T, Suter MR, et al. 2005; Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury. J Neurosci. 25:7317–23. DOI: 10.1523/JNEUROSCI.1526-05.2005. PMID: 16093381. PMCID: PMC6725303. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=23744437312&origin=inward.

6. Vanderah TW, Ossipov MH, Lai J, Malan TP Jr, Porreca F. 2001; Mechanisms of opioid-induced pain and antinociceptive tolerance: descending facilitation and spinal dynorphin. Pain. 92:5–9. DOI: 10.1016/S0304-3959(01)00311-6. PMID: 11323121. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0035063380&origin=inward.

7. Gardell LR, Wang R, Burgess SE, Ossipov MH, Vanderah TW, Malan TP Jr, et al. 2002; Sustained morphine exposure induces a spinal dynorphin-dependent enhancement of excitatory transmitter release from primary afferent fibers. J Neurosci. 22:6747–55. DOI: 10.1523/JNEUROSCI.22-15-06747.2002. PMID: 12151554. PMCID: PMC6758163. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0036703883&origin=inward.

8. Yamamoto Y, Ueta Y, Date Y, Nakazato M, Hara Y, Serino R, et al. 1999; Down regulation of the prepro-orexin gene expression in genetically obese mice. Brain Res Mol Brain Res. 65:14–22. DOI: 10.1016/S0169-328X(98)00320-9. PMID: 10036303. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0033582606&origin=inward.

9. Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, et al. 1999; The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 98:365–76. DOI: 10.1016/S0092-8674(00)81965-0. PMID: 10458611. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0033529520&origin=inward.

10. Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, et al. 1999; Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci U S A. 96:748–53. DOI: 10.1073/pnas.96.2.748. PMID: 9892705. PMCID: PMC15208. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0033582175&origin=inward.

11. Jeon Y, Park KB, Pervin R, Kim TW, Youn DH. 2015; Orexin-A modulates excitatory synaptic transmission and neuronal excitability in the spinal cord substantia gelatinosa. Neurosci Lett. 604:128–33. DOI: 10.1016/j.neulet.2015.08.001. PMID: 26254164. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84939240469&origin=inward.

12. Chen J, Sandkühler J. 2000; Induction of homosynaptic long-term depression at spinal synapses of sensory a delta-fibers requires activation of metabotropic glutamate receptors. Neuroscience. 98:141–8. DOI: 10.1016/S0306-4522(00)00080-4. PMID: 10858620. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0342979863&origin=inward.

13. Park KB, Weon H. 2017; Orexin receptors mediate long-term depression of excitatory synaptic transmission in the spinal cord dorsal horn. Neurosci Lett. 660:12–6. DOI: 10.1016/j.neulet.2017.08.068. PMID: 28866050. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85029174995&origin=inward.

14. Niknia S, Kaeidi A, Hajizadeh MR, Mirzaei MR, Khoshdel A, Hajializadeh Z, et al. 2019; Neuroprotective and antihyperalgesic effects of orexin-A in rats with painful diabetic neuropathy. Neuropeptides. 73:34–40. DOI: 10.1016/j.npep.2018.11.001. PMID: 30447858. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85056692005&origin=inward.

15. Yamamoto T, Saito O, Shono K, Aoe T, Chiba T. 2003; Anti-mechanical allodynic effect of intrathecal and intracerebroventricular injection of orexin-A in the rat neuropathic pain model. Neurosci Lett. 347:183–6. DOI: 10.1016/S0304-3940(03)00716-X. PMID: 12875916. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0043091918&origin=inward.

16. Risold PY, Griffond B, Kilduff TS, Sutcliffe JG, Fellmann D. 1999; Preprohypocretin (orexin) and prolactin-like immunoreactivity are coexpressed by neurons of the rat lateral hypothalamic area. Neurosci Lett. 259:153–6. DOI: 10.1016/S0304-3940(98)00906-9. PMID: 10025581. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0033555609&origin=inward.

17. Araldi D, Ferrari LF, Levine JD. 2015; Repeated mu-opioid exposure induces a novel form of the hyperalgesic priming model for transition to chronic pain. J Neurosci. 35:12502–17. DOI: 10.1523/JNEUROSCI.1673-15.2015. PMID: 26354917. PMCID: PMC4563038. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84941247747&origin=inward.

18. Yaksh TL, Rudy TA. 1976; Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 17:1031–6. DOI: 10.1016/0031-9384(76)90029-9. PMID: 14677603. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0017036162&origin=inward.

19. Deuis JR, Dvorakova LS, Vetter I. 2017; Methods used to evaluate pain behaviors in rodents. Front Mol Neurosci. 10:284. DOI: 10.3389/fnmol.2017.00284. PMID: 28932184. PMCID: PMC5592204. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85032342324&origin=inward.

20. Charan J, Kantharia ND. 2013; How to calculate sample size in animal studies? J Pharmacol Pharmacother. 4:303–6. DOI: 10.4103/0976-500X.119726. PMID: 24250214. PMCID: PMC3826013. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84886544464&origin=inward.

21. Wang Z, Gardell LR, Ossipov MH, Vanderah TW, Brennan MB, Hochgeschwender U, et al. 2001; Pronociceptive actions of dynorphin maintain chronic neuropathic pain. J Neurosci. 21:1779–86. DOI: 10.1523/JNEUROSCI.21-05-01779.2001. PMID: 11222667. PMCID: PMC6762963. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0035282798&origin=inward.

22. Vanderah TW, Laughlin T, Lashbrook JM, Nichols ML, Wilcox GL, Ossipov MH, et al. 1996; Single intrathecal injections of dynorphin A or des-Tyr-dynorphins produce long-lasting allodynia in rats: blockade by MK-801 but not naloxone. Pain. 68:275–81. DOI: 10.1016/S0304-3959(96)03225-3. PMID: 9121815. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=17344385566&origin=inward.

23. Mobarakeh JI, Takahashi K, Sakurada S, Nishino S, Watanabe H, Kato M, et al. 2005; Enhanced antinociception by intracerebroventricularly and intrathecally-administered orexin A and B (hypocretin-1 and -2) in mice. Peptides. 26:767–77. DOI: 10.1016/j.peptides.2005.01.001. PMID: 15808907. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=16244381170&origin=inward.

24. Cheng JK, Chou RC, Hwang LL, Chiou LC. 2003; Antiallodynic effects of intrathecal orexins in a rat model of postoperative pain. J Pharmacol Exp Ther. 307:1065–71. DOI: 10.1124/jpet.103.056663. PMID: 14551290. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0344304799&origin=inward.

25. Araldi D, Ferrari LF, Levine JD. 2017; Hyperalgesic priming (type II) induced by repeated opioid exposure: maintenance mechanisms. Pain. 158:1204–16. DOI: 10.1097/j.pain.0000000000000898. PMID: 28306605. PMCID: PMC5474187. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85021436988&origin=inward.

26. de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. 1998; The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 95:322–7. DOI: 10.1073/pnas.95.1.322. PMID: 9419374. PMCID: PMC18213. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0008390266&origin=inward.
27. Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. 1998; Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 92:573–85. DOI: 10.1016/S0092-8674(00)80949-6. PMID: 9527442. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=20244380014&origin=inward.

28. Cutler DJ, Morris R, Sheridhar V, Wattam TA, Holmes S, Patel S, et al. 1999; Differential distribution of orexin-A and orexin-B immunoreactivity in the rat brain and spinal cord. Peptides. 20:1455–70. DOI: 10.1016/S0196-9781(99)00157-6. PMID: 10698122. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0033370580&origin=inward.

29. van den Pol AN. 1999; Hypothalamic hypocretin (orexin): robust innervation of the spinal cord. J Neurosci. 19:3171–82. DOI: 10.1523/JNEUROSCI.19-08-03171.1999. PMID: 10191330. PMCID: PMC6782271. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0033561178&origin=inward.

30. Bingham S, Davey PT, Babbs AJ, Irving EA, Sammons MJ, Wyles M, et al. 2001; Orexin-A, an hypothalamic peptide with analgesic properties. Pain. 92:81–90. DOI: 10.1016/S0304-3959(00)00470-X. PMID: 11323129. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0035061724&origin=inward.

31. Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA. 2001; Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience. 103:777–97. DOI: 10.1016/S0306-4522(01)00033-1. PMID: 11274794. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0035925698&origin=inward.

32. Kimura M, Kawai Y, Ishikawa M, Shibutani K, Uchida R, Eguchi Y, et al. 2014; Effects of lidocaine and orexin on [Ca2+]i in dosal root ganglion neuron of rat segmental spinal nerve ligation model. Jpn Pharmacol Ther. 42:723–34. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0035925698&origin=inward.
33. Zhou HY, Chen SR, Chen H, Pan HL. 2010; Opioid-induced long-term potentiation in the spinal cord is a presynaptic event. J Neurosci. 30:4460–6. DOI: 10.1523/JNEUROSCI.5857-09.2010. PMID: 20335482. PMCID: PMC2852319. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=77949839792&origin=inward.

34. Watanabe S, Kuwaki T, Yanagisawa M, Fukuda Y, Shimoyama M. 2005; Persistent pain and stress activate pain-inhibitory orexin pathways. Neuroreport. 16:5–8. DOI: 10.1097/00001756-200501190-00002. PMID: 15618879. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=13244273392&origin=inward.

35. Azhdari-Zarmehri H, Semnanian S, Fathollahi Y. 2015; Orexin-A modulates firing of rat rostral ventromedial medulla neurons: an in vitro study. Cell J. 17:163–70. DOI: 10.22074/cellj.2015.524. PMID: 25870847. PMCID: PMC4393666.
36. Bannister K, Lee YS, Goncalves L, Porreca F, Lai J, Dickenson AH. 2014; Neuropathic plasticity in the opioid and non-opioid actions of dynorphin A fragments and their interactions with bradykinin B2 receptors on neuronal activity in the rat spinal cord. Neuropharmacology. 85:375–83. DOI: 10.1016/j.neuropharm.2014.06.005. PMID: 24937046. PMCID: PMC4873257. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84903195985&origin=inward.

37. Kajander KC, Sahara Y, Iadarola MJ, Bennett GJ. 1990; Dynorphin increases in the dorsal spinal cord in rats with a painful peripheral neuropathy. Peptides. 11:719–28. DOI: 10.1016/0196-9781(90)90187-A. PMID: 1978300. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0025142527&origin=inward.

38. Vanderah TW, Gardell LR, Burgess SE, Ibrahim M, Dogrul A, Zhong CM, et al. 2000; Dynorphin promotes abnormal pain and spinal opioid antinociceptive tolerance. J Neurosci. 20:7074–9. DOI: 10.1523/JNEUROSCI.20-18-07074.2000. PMID: 10995854. PMCID: PMC6772839. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0034666274&origin=inward.

39. Yamamoto T, Nozaki-Taguchi N, Chiba T. 2002; Analgesic effect of intrathecally administered orexin-A in the rat formalin test and in the rat hot plate test. Br J Pharmacol. 137:170–6. DOI: 10.1038/sj.bjp.0704851. PMID: 12208773. PMCID: PMC1573477. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0036740492&origin=inward.

Fig. 1
Repeated exposure to [D-Ala2, N-Me-Phe4, Gly5-ol]-enkephalin (DAMGO) induced mechanical hypersensitivity in rats. Repetitive intradermal injection (4 times, at 1-hour intervals) of DAMGO (5 µg/5 µL) into the hind paw dorsum significantly decreased mechanical threshold measured by von Frey filament test (g) on the day of and 4 and 6 days after injection. The error bars indicate SEM. **P < 0.01 vs. saline control; #P < 0.05 and ##P < 0.01 vs. baseline (i.e., before the DAMGO injection).
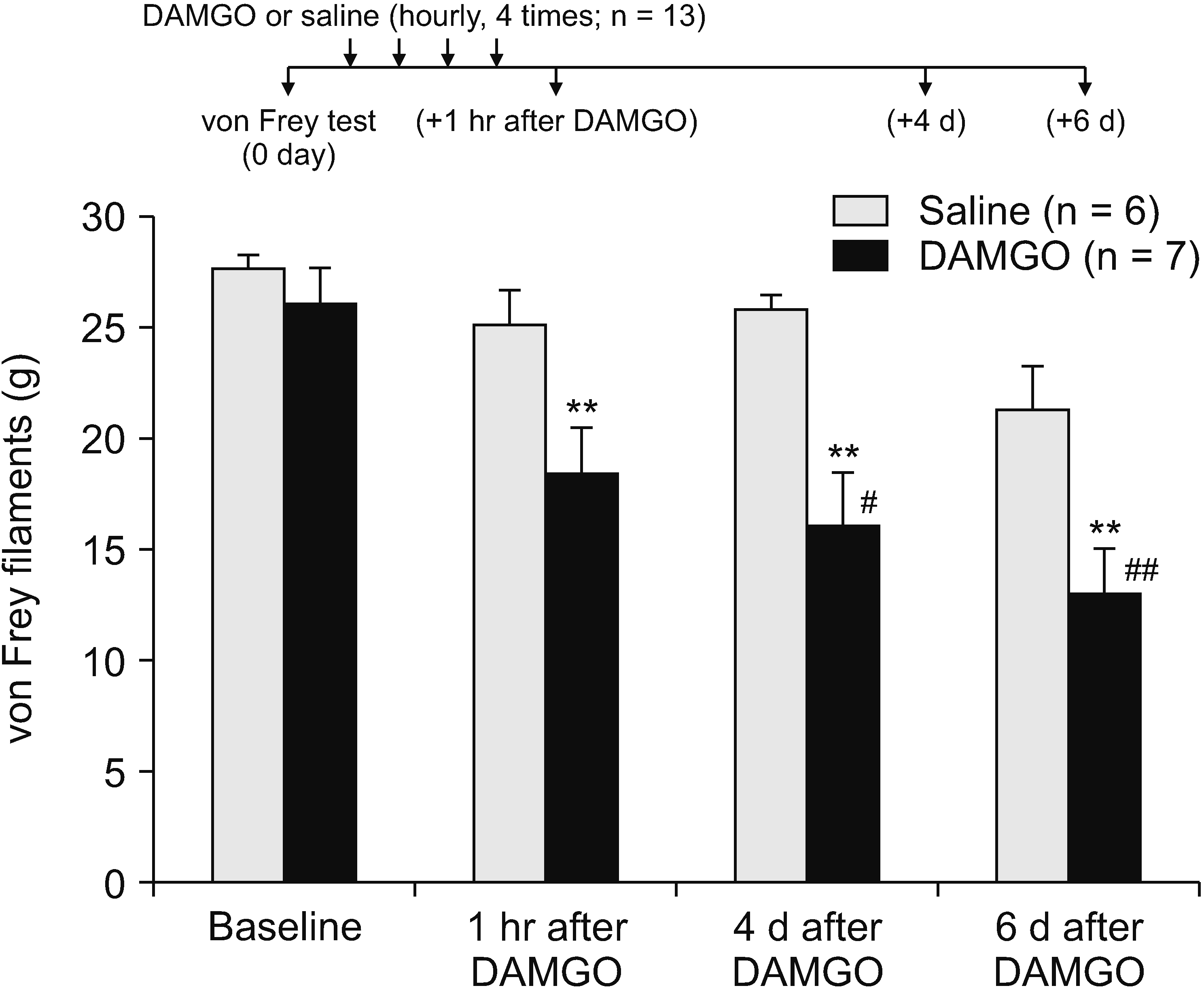
Fig. 2
Spinal orexin A attenuated [D-Ala2, N-Me-Phe4, Gly5-ol]-enkephalin (DAMGO)-induced mechanical hypersensitivity. Intrathecal administration of orexin A (5 µg/5 µL, i.t.) normalized the mechanical hypersensitivity that was developed on the day of and lasted until 8 days after repetitive intradermal injection (4 times, at 1-hour intervals) of DAMGO on the rat hind paw dorsum, although the first intrathecal administration of orexin A (6 days post DAMGO) had no effect. The effect of orexin A was inhibited by SB674042 (5 µg/5 µL), the OX1 receptor antagonist. The error bars indicate SEM. *P < 0.05 vs. baseline of each group; #P < 0.05 vs. the thresholds (g) of other four groups at 8 days after intradermal DAMGO injection. An experimental scheme above the histogram is shown with the number of rats used vs. the number of rats subjected with catheter insertion (n = 31/33). OX1: type 1 orexin, DMSO: dimethyl sulfoxide.
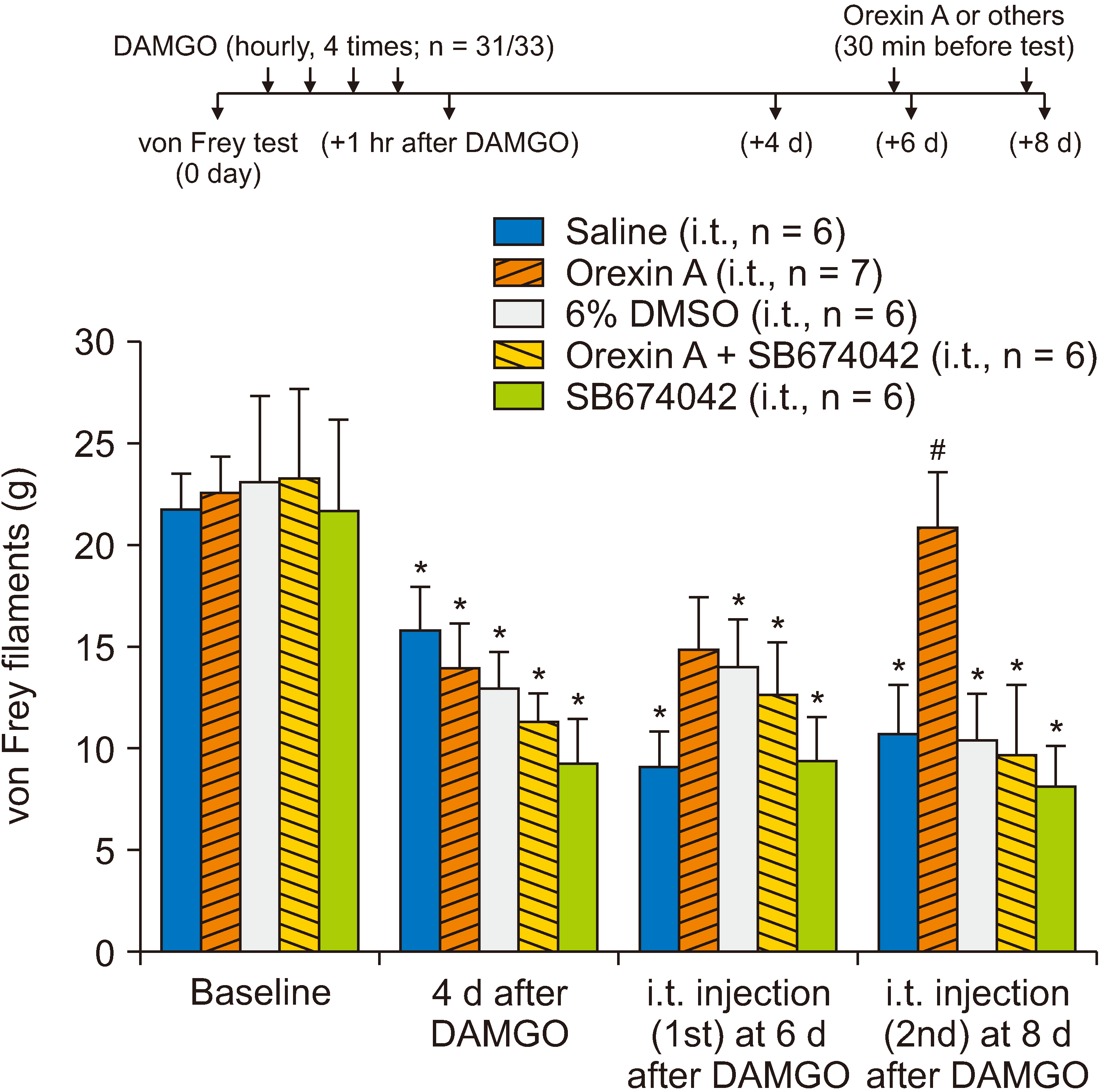
Fig. 3
Spinal dynorphin or preventing spinal endogenous dynorphin signals had no effect on the [D-Ala2, N-Me-Phe4, Gly5-ol]-enkephalin (DAMGO)-induced mechanical hypersensitivity. The mechanical hypersensitivity induced by repetitive intradermal injection of DAMGO (4 times, at 1-hour intervals) was not affected by intrathecal administration (i.t.) of dynorphin (5 µg/5 µL) or antiserum to dynorphin (5 µg/5 µL) at 6 and 8 days after the DAMGO exposure. The error bars indicate SEM. *P < 0.05 vs. baseline. An experimental scheme above the histogram is shown with the number of rats used vs. the number of rats subjected with catheter insertion (n = 18/23).
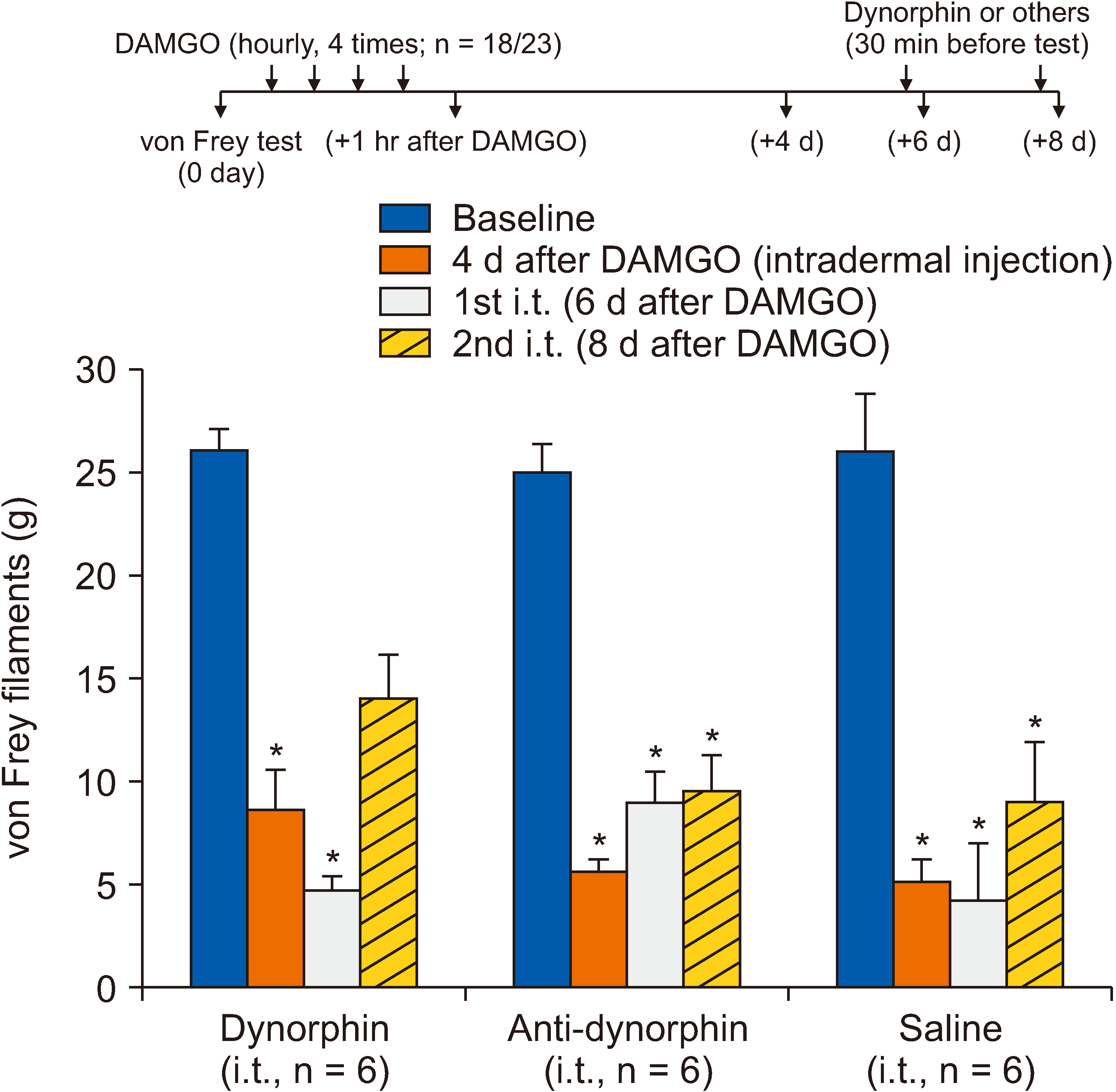
Fig. 4
Pre-dosing of orexin A or dynorphin did not prevent the induction of mechanical hypersensitivity by repeated exposure of [D-Ala2, N-Me-Phe4, Gly5-ol]-enkephalin (DAMGO). The mechanical hypersensitivity was induced by four times of repetitive intradermal injection of DAMGO (hourly). Orexin A (5 µg/5 µL), dynorphin (5 µg/5 µL), antiserum to dynorphin (5 µg/5 µL), or saline were intrathecally administered just before the DAMGO exposure. The error bars indicate SEM. **P < 0.01 vs. baseline mechanical sensitivity before DAMGO exposure. An experimental scheme above the histogram is shown with the number of rats used vs. the number of rats subjected with catheter insertion (n = 32/35). i.t.: intrathecal administration.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download