INTRODUCTION
Clinical research shows that the incidence of postoperative pain is about 10%–50%, of which 10% of patients still suffer from chronic pain even if the primary disease has been cured. The authors define it as chronic post-surgical pain (CPSP) [
1]. There is no effective treatment for CPSP because the mechanism is unclear [
2]. Various perioperative stressors, such as anxiety, noise, insomnia, and movement restrictions, may induce stress responses that correlate with CPSP [
3–
5].
Opioid receptors, which can be divided into four subtypes: μ, κ, δ and σ, are widely distributed in the central nervous system. The distribution concentrations, from high to low, are the cerebral cortex, amygdala, septal nucleus, thalamus, hypothalamus, midbrain, and spinal cord. Endogenous or exogenous opioid peptides act on the opioid receptors, which are coupled with C protein, inhibit the activity of adenylate cyclase and calcium channels, activate the potassium channels, reduce the reactivity of neurons, and produce an analgesic effect [
5–
7].
The amygdala is considered to be the center of the comprehensive processing of negative emotional information and pain perception [
8,
9]. Studies have shown that anxiety induced by chronic pain is accompanied by changes in the expression of mu-opioid receptors (MOR) in the amygdala, which affects the generation and maintenance of chronic pain [
10,
11]. The decreased expression of MOR in amygdala was closely related to CPSP.
The upstream molecular mechanism of CPSP may be related to epigenetic alterations [
12–
14]. MicroRNA (miRNA) is a kind of noncoding single-stranded RNA molecule with a length of about 20–25 nucleotides encoded by endogenous genes. They participate in the regulation of post transcriptional gene expression [
15,
16]. When a miRNA binds its target mRNA completely, the target mRNA is immediately degraded. However, if they do not fully bind, the miRNA inhibits the translation of the target mRNA. miRNAs play a critical role in the regulation of all biological functions such as development, cell proliferation, cell differentiation, and apoptosis [
17]. For their biological functions, miRNAs are associated with many human pathologies such as malignant tumors, heart disease, diabetes, autoimmune diseases, inflammatory diseases, and Alzheimer’s disease [
18,
19]. In recent years, researchers have found that certain miRNAs are causally involved in the development of chronic postoperative pain [
20,
21].
In the previous study, it was found the expression of miRNA-339-5p was increased while the expression of MOR in the amygdala of a perioperative stress animal model was decreased. The authors focused on whether miR-339-5p participated in CPSP. It was hypothesized that miR-339-5p could regulate the expression of MOR via targeting opioid receptor mu 1 (oprm1), then participating in CPSP.
Go to :

MATERIALS AND METHODS
1. Animal preparation and model building
Adult male Sprague-Dawley rats, weighing 180–200 g, were purchased from Changsheng Biotechnology Co. Ltd., Liaoning Province, China. The rats were housed in individual cages with free access to water and food. All rats lived with a 12-hour day-night cycle at a room temperature of 20°C–26°C and a relative humidity of 40%–70%. The method for anesthesia was 10% chloral hydrate administered with an intraperitoneal injection at the dose of 0.004 mL/g body weight. The animal experiments were performed according to the Guide for the Care and Use of Laboratory Animals and approved by the Animal Investigation Committee of General Hospital of Southern Theatre Command of PLA (approval number: IACU19-0320). To build a perioperative stress prolongs incision-induced pain hypersensitivity without changing basal pain perception rat model, there was a need to determine how many days the stress should last. Ten rats were randomized into a stress group and a control group (n = 5). The rats in the stress group were woken up when they were asleep, then wrapped tightly in barbed wire, restricted their activities without water and food for 6 hours. They were kept awake during this period. The control group was not treated. Both groups were given a mechanical pain, cold withdrawal latency, and thermal withdrawal latency test daily for 7 days.
Another 40 rats were randomized into 4 groups (n = 10). The rats in each group were treated as described in
Table 1.
Table 1
The experimental grouping and treatment of the rats
|
Group |
Abbreviation |
Number |
Treatment |
|
Control |
sham |
10 |
Rats were anesthetized without any other treatment |
|
Incision |
IN |
10 |
After anesthesia, a 1 cm incision was made on the rats’ plantar aspect of left hind paw and separated the tendon of the sole bluntly with hemostatic forceps, hemostasis and sutured |
|
Stress |
S |
10 |
Rats were anesthetized without surgery. Waked up the rats after they fell asleep, then wrapped them tightly by barbed wire, restricted their activities without water and food for 6 hr from 9:00 a.m. to 3:00 p.m. kept them awaked during this period. Repeat these steps daily for 3 daysa
|
|
Stress + Incision |
S + IN |
10 |
After anesthesia, perform an operation as same as group IN, stressed as same as group S for 3 daysa from the day taken operation |

To evaluate the recovery time of postoperative pain in each group of rats after stress. The pain behavior of rats to mechanical, thermal, and cold stimuli were measured 1 day before the operation, and on days 1, 3, 5, 7, and 9 after the operation.
2. pmiR-RB-REPORTTM Double luciferase assay to verify that miR-339-5p could act on oprm1 as a target
The reporter fluorescence of the vector in pmiR-RB-REPORTTM Double luciferase assay (Promega, Madison, WI) is the renilla luciferase gene (hRluc), and the corrected fluorescence is the firefly luciferase gene (hluc, as an internal reference). The 3’untranslated region (3’UTR) region of oprm1 is cloned at the hrluc gene downstream. The miRNA acts on the target gene through the 3’UTR region. Therefore, the miR-339-5p was co-transformed with the constructed reporter gene vector. The interaction between miRNA and the target gene was confirmed by the down-regulation of the relative fluorescence value of the reporter gene. Confirmation of the interaction between miR-339-5p and oprm1 was proved by the down-regulation of the relative fluorescence value of the reporter gene.
The 3’UTR of
oprm1 containing the miR-339-5p binding site was amplified with the forward primer and the reverse primer (Ribobio, Guangzhou, China) from rat genomic DNA samples. The polymerase chain reaction (PCR) products were separated in an agarose gel and extracted, purified, and cloned using the Seamless Cloning Kit (Beyotime, Shanghai, China). The
oprm1 3’UTR was linked to the vector pmiR-RB-Report
TM (Ribobio) containing renilla and firefly luciferase using the restriction enzymes XhoI and NotI (information of the reporter gene vector seen in
Table 2). The resulting construct was verified by sequencing. 293T cells were seeded at 1 × 10
4 cells per well in 96-well plates. The cells were transfected using Lipo6000
TM (Beyotime) according to the manufacturer’s suggestions 24 hours after plating. In each well, 50 ng of pmiR-RB-Report
TM-3’UTR vector or Mut-3’UTR vector was co-transfected with 50 nM micrONTM rno-miR-339-5p (Ribobio), while 50 nM micrONTM miRNA mimic Negative Control #24 was used in each experiment. Three replicates of each sample were evaluated, and each experiment was repeated at least three times. The cells were harvested in 70 μL of passive lysis buffer per well 48 hours after transfection. The activity of renilla luciferase in the cell lysate was measured using the Dual-Glo
® Luciferase Assay System (Promega Biotech Co., Ltd, Beijing, China) in a GloMax
® 96 Microplate Luminometer (Promega) and normalized to the activity of firefly luciferase.
Table 2
Informations of pmiR-RB-REPORTTM Double luciferase assay reporter gene vector
|
Name of sequences |
r-Oprm1(NM_001304733.1 complete sequence)-WT, r-Oprm1(NM_001304733.1 complete sequence)-MUT |
|
Clone length |
902 bp (NM_001304733.1 complete sequence) |
|
5′cleavage site |
XhoI |
|
3′cleavage site |
NotI |
|
Name of vector |
pmiR-RB-ReportTM
|
|
Size of vector’ skeleton |
6.3 kb |
|
Reporter gene |
Renilla luciferase |
|
Reference gene |
Firefly luciferase |
|
Host bacteria |
Escherichia coli DH5α |

3. Mechanical pain threshold and thermal, cold withdrawal latency test
The mechanical pain threshold test used Von Frey filaments (North Coast Medical Inc., Morgan Hill, CA), pricking the plantar aspect of the rat’s hind paw with cilia of different strength from weak to strong (1.0, 1.4, 2.0, 4.0, 6.0, 8.0, 10, 15, and 26 g). If the rat flinched the paw rapidly or licked the paw, it was recorded as positive. If there was no response, it was recorded as negative. The stimulation intensity of cilia was recorded when a positive reaction appeared.
Thermal withdrawal latency test used a hot and cold plate pain meter (BW-YLS-21A; Shanghai Biowill Co., Ltd., Shanghai, China). The temperature of metal plate was adjusted to 55°C. The rats were placed on the metal surface at 55°C and the rat’s locomotion was restricted by a plexiglass cylinder. The time was started when the rats were placed and stopped when the rats flinched their paw rapidly or licked the paw. This time was defined as thermal withdrawal latency.
The cold withdrawal latency test used a hot and cold plate pain meter (BW-YLS-21A; Shanghai Biowill Co.). The temperature of the metal plate was adjusted to 4°C. The rats were placed on the metal surface at 4°C and the rat’s locomotion was restricted by a Plexiglass cylinder. The time was started when the rats were placed and stopped when the rats flinch their paw rapidly or licked their paw. This time was defined as cold withdrawal latency.
4. Test of rats’ serum glucocorticoid
After anesthesia with 10% chloralhydrate, a sterile glass capillary was inserted into the sinus vein plexus of the rat. About 500 μL of blood was collected and centrifuged at 4°C/4,000 rpm for 10 minutes, and the serum stored at –80°C. Corticosterone levels were tested using enzyme-linked immunosorbent assay (USCN Co., Ltd., Wuhan, China).
5. Separation of the rat’s amygdala
The rats were euthanized after anesthesia, and the whole brain was separated and put on ice immediately. The amygdala was isolated under a microscope and stored at –80°C.
6. Immunofluorescence
The amygdalas of the rats were fixed with 4% paraformaldehyde for 24 hours. After dehydration, the amygdalas were sliced into 4 μm thick sections. Slices were put into phosphate buffered saline (PBS) for 3 minutes, repeated 3 times. Each slice was added with 200 uL of 5% bovine serum albumin (BSA) and blocked for 30 minutes. Incubated with primary antibodies diluted in blocking solution at 4°C overnight (Anti-Mu Opioid Receptor Antibody; Cohesion Biosciences, London, UK, catalog number CPA3285, 1:500). Then, sections were washed four times with PBS (0.01 M) and incubated with secondary antibodies (fluorescein isothiocyanate conjugated sheep anti-rabbit antibody; Abcam Inc., Cambridge, UK, catalog number ab7086, 1:400), incubated in a 37°C oven for 30 minutes, and washed again four times with phosphate buffered solution with tween-20. Diluted 4’,6-diamidino-2-phenylindole was added and incubated at room temperature for 10 minutes. Images were taken with a laser-scanning confocal microscope (Fluoview FV1000; Olympus, Tokyo, Japan) and processed with Image J (National Institutes of Health, Bethesda, MD) and photoshop CS3 (Abode, San Jose, CA) software.
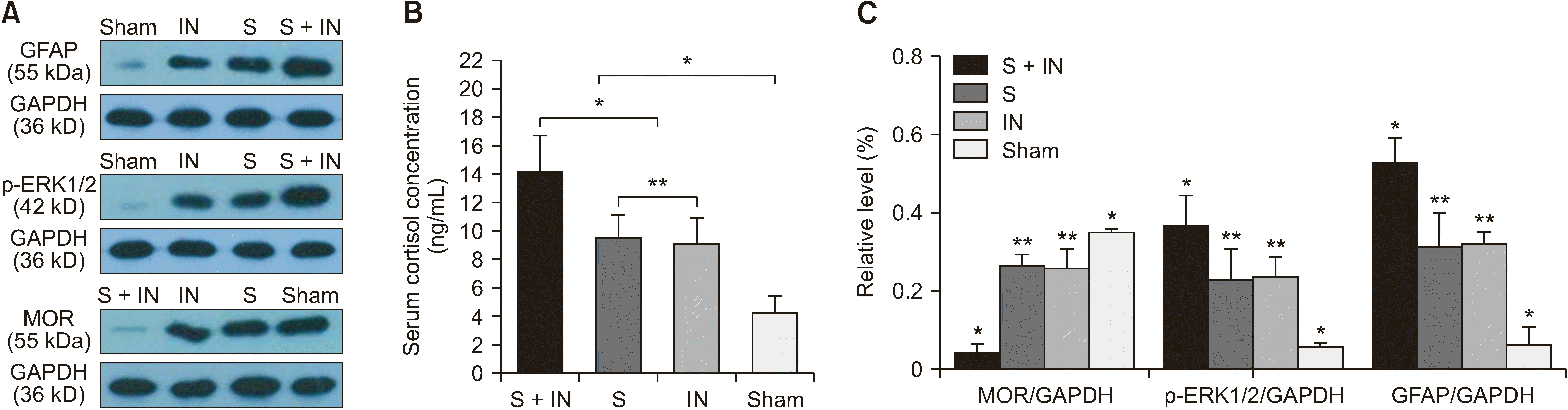
7. Western blotting
The proteins were extracted using tissue protein extraction reagent (Webiao Biotech, Shanghai, China). Protein concentrations were determined with a Pierce BCA Protein Assay Kit (Beyotime). The proteins were transferred to a polyvinylidene difluoride membrane. The membranes were blocked with a 5% milk solution prepared in PBS for 60 minutes. Then incubated at 4°C overnight with 1:1,000 diluted primary antibody (Anti-Mu Opioid Receptor Antibody: Cohesion Biosciences, catalog number CPA3285; Anti-phosphorylated extracellular regulatory protein kinase [p-ERK1/2] Antibody: Cohesion Biosciences, catalog number CPA4924; Anti-glial fibrillary acidic protein [GFAP] Antibody: Cohesion Biosciences, catalog number CPA4418). The membranes were washed three times for 5 minutes each with Tween 20 (1:1,000 dilution in PBS), then incubated for 45 minutes with the appropriate peroxidase-conjugated secondary antibody (1:5,000 dilution). The membranes were washed three times with Tween 20-PBS and were developed using an Odyssey two-color infrared laser imaging system (LI-COR, Lincoln, NE). The signal generated by GADPH was used as an internal control.
8. Immunohistochemistry staining
The paraffin-embedded sections of rat amygdala tissue were immersed in 3% hydrogen peroxide solution for 10 minutes to block the endogenous peroxidase after it was de-paraffinized and rehydrated using xylene and ethanol. Sections were boiled for 30 minutes in 10 mM citrate buffer solution (pH 6.0) for antigen retrieval. Slides were incubated for 45 minutes with 5% BSA and incubated overnight at 4°C with Anti-p-ERK1/2 antibody (Cohesion Biosciences, catalog number CPA4924, 1:500), Anti-GFAP antibody (Cohesion Biosciences, catalog number CPA4418, 1:200) and Anti-Mu Opioid Receptor antibody (Cohesion Biosciences, catalog number CPA3285, 1:500). The specimens were incubated for 45 minutes at 37°C with the appropriate peroxidase-conjugated secondary antibody and visualized using the DAB Detection Kit (BOSTER, Shanghai, China) following the manufacturer’s instructions. All sections with immunohistochemical staining were observed and the pictures of 6 randomly selected fields (but which were homogeneous in staining and cell numbers) (×200) were photographed by an Olympus microscope (CK31; Olympus) under high power view. The integrated optical density in each image was measured with the same setting for all the slides, and the results were analyzed with Image-ProPlus 6.0 software (Media Cybernetics, Rockville, MD).
9. RNA isolation and quantitative real-time PCR (qRT-PCR)
Trizol was used to extract total RNA from the rat amygdala according to the instructions of the kit (Tiangen Biochemical Technology Co., Ltd, Beijing, China). The RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA) was used to synthesis complementary DNA. qRT-PCR was performed using the LightCycler®480 II system (Roche, Basel, Switzerland). The FastStart Universal SYBR Green Master kit (Roche) was used to detect MOR mRNA with the following PCR amplification cycles: initial denaturation, 95°C for 5 minutes followed by 44 cycles with denaturation at 95°C for 15 seconds, annealing at 65°C for 30 seconds, and extension at 72°C for 20 seconds. B-actin was used as internal references for both MOR mRNA and miR-339-5p. The relative level of expression was calculated using the comparative (2Ct) method. All samples were evaluated in triplicate. The primer sequences were as follows:
U6snRNA-F: 5’-CTCGCTTCGGCAGCACA-3’
U6snRNA-R: 5’-AACGCTTCACGAATTTGCGT-3’
miR-339-5p-F: 5’-ATGATCCCTGTCCTCCAGGA-3’
miR-339-5p-R: 5’-GTGCAGGGTCCGAGGTATTC-3’
10. Statistics
The data are presented as the mean ± SEM. GraphPad Prism v8.0 (GraphPad Software, San Diego, CA) was used for statistical analyses. The normality of the data was assessed using the Shapiro–Wilk and Kolmogorov–Smirnov tests, with P < 0.050 accepted as a non-normal distribution. Equality of variance was assessed using an F test. An unpaired Student’s t-test was used for normally distributed datasets. Comparisons of a non-normally distributed group with any other group were made using the Mann–Whitney U-test. Comparisons of more than two groups, all of which were normally distributed, were made using one-way analysis of variance with Tukey’s post-hoc tests for pairwise comparisons. The data from behavior test were statistically analyzed with two-way analysis of variance. P < 0.05 was considered significant.
Go to :

DISCUSSION
CPSP refers to chronic pain that is present for at least 3 months after a surgical procedure [
22]. The mechanism of CPSP is still unclear. Some clinical research shows that peri-operative patients may suffer interference from stress, anxiety, environmental noise during sleep, and restrictions of movements which can affect the central nervous system of patients that lead to endocrine disorders [
23].
Opioid receptors are distributed in the central nervous system. The concentration of opioid receptors in the amygdala is second only to that in cerebral cortex. Studies have shown that the expression of MOR in the amygdala is negatively correlated with the generation and persistence of chronic pain [
12–
14]. The amygdala is considered to be the center of the comprehensive processing of negative emotional information and pain perception. Therefore, down-expression of MOR in the amygdala might be the key central molecular mechanism of CPSP [
24]. However, the exact molecular mechanism of its upstream regulation is still unknown.
In recent years, more and more attention has been paid to the relationship between epigenetics and CPSP [
25]. Perioperative stress could bring about DNA methylation, histone modification, and non-coding RNAs expressions. These changes may be related to pain hypersensitivity under chronic pain conditions [
26,
27].
miRNAs exist extensively
in vivo and participate in many physiological and pathological processes in which they alter and modulate the expression of proteins [
17–
19]. miR-339-5p often plays the role of a tumor suppressor in cancer, and is associated with inhibition of esophageal cancer, pancreatic cancer, lung cancer and ovarian cancer [
28–
31]. There are few reports about miR-339-5p and chronic pain. In a previous study, the authors found that miR-339-5p directly targets
oprm1 by using RNAhybrid software, and the expression of miR-339-5p increased while the expression of MOR decreased in the amygdala of rats. Therefore, it was hypothesized that perioperative physical stress, psychological stress, and surgical trauma stimulation causes emotional tension, sleep disorders, and movements restriction, which lead to neuroendocrine disorders, thus promoting an increase in the expression of miR-339-5p in the amygdala while the expression of MOR decreased, finally leading to the occurrence of CPSP.
In order to verify these hypotheses, an appropriate animal model was needed. According to the pre-experimental results, sleep deprivation and movement restriction did not change the basic pain threshold of rats in 3 days, but the pain threshold began to decrease at the 4th day. Therefore, the short-term stress time duration of this study was set as 3 days. By detecting the changes of pain behavior in perioperative stress rats, the authors found that short-term perioperative stress could prolonged the duration of incision pain and the time of pain recovery. The results of western blot and immunohistochemistry showed that p-ERK1/2 and GFAP increased in the amygdala of short-term perioperative stress rats. They indicated that the level of spontaneous pain is increased after stress. These demonstrated that the short-term perioperative stress does not affect basal pain perception, but does delay postsurgical pain recovery. The perioperative stress prolong post-surgical pain animal model was constructed successfully, which provided a key tool for this study.
The cortisone level of the blood was increased significantly in the S + IN group compared to the other groups. These results demonstrate that short-term perioperative stress could influence the endocrine system of rats. The stress applied put the rats in a state of stress. Endocrine disorder may be a bridge connecting stress and changes in the expression of miR-339-5p and MOR.
It is known that miRNAs silence genes either by initiating the cleavage of their respective target mRNA after complete binding to their target sequences or by inhibiting gene translation after partial binding to their target sequences [
32]. The authors found that the expression level of MOR protein and mRNA in the amygdala were significantly decreased in the rats of the S + IN group compared with other groups, while the expression level of miR-339-5p was increased in rats of S + IN group. The expression level of MOR protein and mRNA in amygdala were significantly decreased in rats of group S and group IN compared with the sham group, while the expression level of miR-339-5p was increased in rats of the S + IN group. These showed that both perioperative stress and surgical injury could lead to the down-regulation of MOR’ expression and the up-regulation of miR-339-5p’ expression.
Furthermore, this study confirmed that miRNA-339-5p could act directly on oprm1. Using RNAhybrid, it was found that MOR mRNA is a target of miR-339-5p. The results of luciferase assay indicated that the oprm1 3’UTR was in fact targeted by miR-339-5p. miR-339-5p regulates the expression of MOR protein by competing with MOR mRNA. Thus, the authors’ previous hypothesis was verified: perioperative physical stress, psychological stress, and surgical trauma stimulation causes emotional tension and sleep disorders, which lead to neuroendocrine disorders. This may cause the expression of miR-339-5p to increase, thus resulting in the expression of MOR in the amygdala decreasing, finally giving rise to CPSP.
In conclusion, this study demonstrated that the expression of MOR and miR-339-5p was changed in a perioperative stress prolongs post-surgical pain rat model. The expression of miR-339-5p was negatively correlated with the expression of MOR. This study is the first to report that miR-335-5p is involved in CPSP. The authors have not done relevant mechanism experiments to prove how perioperative stress affects the changes of miR-335-5p, so the mechanism remains unclear. But this study can still identify miR-339-5p as a novel potential therapeutic target in CPSP.
Go to :

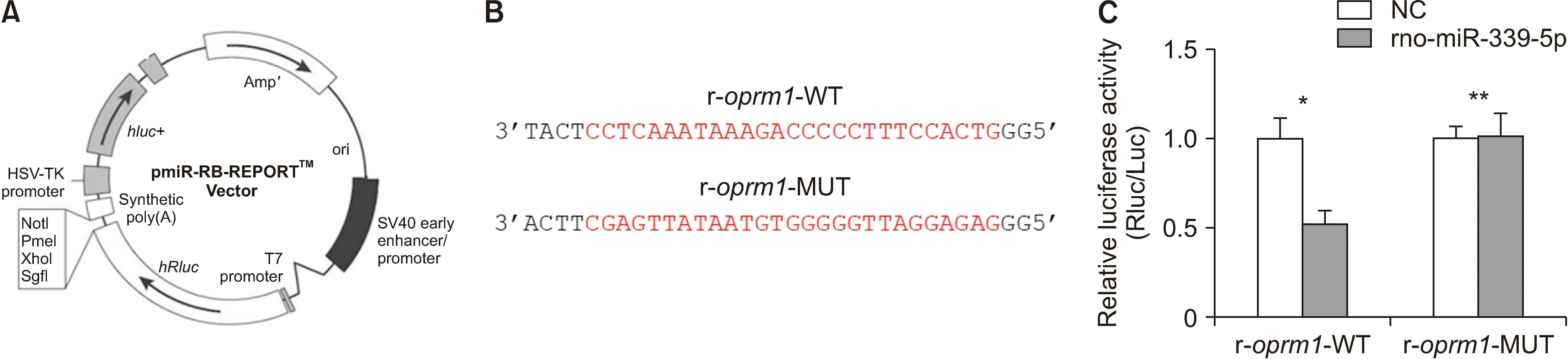






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download