Abstract
Background
The mechanism of peripheral axon transport in neuropathic pain is still unclear. Chemokine ligand 13 (CXCL13) and its receptor (C-X-C chemokine receptor type 5, CXCR5) as well as GABA transporter 1 (GAT-1) play an important role in the development of pain. The aim of this study was to explore the axonal transport of CXCL13/CXCR5 and GAT-1 with the aid of the analgesic effect of botulinum toxin type A (BTX-A) in rats.
Methods
Chronic constriction injury (CCI) rat models were established. BTX-A was administered to rats through subcutaneous injection in the hind paw. The pain behaviors in CCI rats were measured by paw withdrawal threshold and paw withdrawal latencies. The levels of CXCL13/CXCR5 and GAT-1 were measured by western blots.
Results
The subcutaneous injection of BTX-A relieved the mechanical allodynia and heat hyperalgesia induced by CCI surgery and reversed the overexpression of CXCL13/CXCR5 and GAT-1 in the spinal cord, dorsal root ganglia (DRG), sciatic nerve, and plantar skin in CCI rats. After 10 mmol/L colchicine blocked the axon transport of sciatic nerve, the inhibitory effect of BTX-A disappeared, and the levels of CXCL13/CXCR5 and GAT-1 in the spinal cord and DRG were reduced in CCI rats.
Conclusions
BTX-A regulated the levels of CXCL13/CXCR5 and GAT-1 in the spine and DRG through axonal transport. Chemokines (such as CXCL13) may be transported from the injury site to the spine or DRG through axonal transport. Axon molecular transport may be a target to enhance pain management in neuropathic pain.
Neuropathic pain, induced by herpes zoster neuralgia, diabetic peripheral neuropathy, complex regional pain syndrome, etc., is characterized by pricking, burning and electric shock-like severe pain, and is difficult to control effectively and for a long duration [1]. At present, the commonly used drugs to treat neuropathic pain in clinical are ion channel inhibitors, such as pregabalin and gabapentin [2]. Nerve block, pulsed radiofrequency, and spinal cord electrical stimulation are also used to treat intractable neuropathic pain [3,4]. However, there are numerous patients still suffering severe pain because of the poor effect of treatment.
The refractory characteristic of neuropathic pain is related to its complex mechanism. At present, neuropathic pain is mainly caused by the increase and facilitation of ion channels on the cell membrane of painful neurons [5], abnormal nerve discharge [6], sympathetic sprouting [7], and so on. The peripheral mechanism of neuropathic pain was mainly at electrophysiological changes such as abnormal discharge of nerve fibers and activation of ion channels [8,9]. However, whether there are dynamic changes in nerve tracts or fibers in the development of neuropathic pain has not been clearly studied. The authors speculate that there may be other ways of signal transmission between nerve fiber and neuron cells after nerve injury.
Botulinum toxin type A (BTX-A) is a macromolecular protein neurotoxin produced by Botox. Previous research showed that subcutaneous injection of BTX-A performed the effect of antinociception by 25 kDa synaptosomal-associated protein cleavage through retrograde axonal transport to the spinal cord via the sciatic nerve [10]. Based on this phenomenon, the authors supposed if there is axonal transport of pain-causing molecules between a nerve fiber and neurons in peripheral nerve injury.
Pain is regulated by small molecular proteins such as chemokines and neurotransmitters. The authors’ previous study indicated that chemokine ligand 13 (CXCL13) and C-X-C chemokine receptor type 5 (CXCR5) regulated morphine analgesia through p38, ERK, and AKT signaling pathways in bone cancer pain rats [11]. The function of GABA transporter 1 (GAT-1) is to reuptake extracellular GABA to maintain the transmission of GABA signals. In pathophysiological pain states, GABA signaling can be blocked due to a decrease in extracellular GABA synthesis and increasing extracellular GABA reuptake via GAT-1 [12].
In this study, the authors suppose that the known pain-causing molecules CXCL13, CXCR5, and GAT-1 may be transported from the nerve injury site to the dorsal root ganglia (DRG) neuron cell body or spinal cord through axon transport after the nerve is injured. Therefore, with the treatment of BTX-A, the axon transport mode between the nerve injury site and the neuron cell body was explored using the cytokines CXCL13, CXCR5, and GAT-1 as research targets.
The animals in this experiment were specific pathogen-free male Sprague–Dawley rats weighing 220–250 g, purchased from Zhengzhou University Experimental Animal Center, Zhengzhou, China. The rats were placed in a clean animal house, which alternated between 12-hour light and 12-hour dark, and were given enough feed and drinking water. The animal room was kept at a constant temperature (22°C ± 0.5°C) and humidity (40%–60%). All animal procedures were performed in accordance with the guidelines of the National Institutes of Health Laboratory Animal Care and Use Guidelines and the Pain Research Guidelines from International Association for the Study of Pain and approved by the Animal Care and Use Committee of Zhengzhou University. Rats were allowed to adapt for about 7 days before surgery and behavioral testing. In this experiment, all animals were randomly grouped.
We established the CCI model according to a previous study [13]. Rats were anesthetized with 10% Chloral hydrate (3–5 mL/kg, intraperitoneal injection). The left hind limb of each rat was shaved and disinfected with an iodophor. The sciatic nerves on the left side were exposed by making a skin incision and blunt dissection through the connective tissue between the gluteus superficialis and biceps femoris muscles. Then the sciatic nerves were separated by a glass needle. Four ligatures are tied loosely around the sciatic nerve at 1 mm intervals with 4-0 surgical wire, to occlude but not arrest epineural blood flow. The incisions were closed with sutures and the rats were placed in their cages. The sham surgery was implemented using a similar procedure without ligation.
Mechanical paw withdrawal thresholds (PWTs) were carried out by a series of calibrated manual Von Frey’s filaments (Stoelting, Kiel, WI), ranging from 0.2 to 15 g. Rats were placed in opaque plastic cages with a wire mesh floor for 30 minutes to adjust to the environment before the test. A Von Frey filament was applied perpendicularly to the plantar surface of the hind paw for 5 seconds. Abrupt paw withdrawal, licking, or shaking were considered to be positive responses during the application of the stimulus or after removal of the filament. The time interval before the application of the next filament was at least 10 seconds. The “up-down” method was used to calculate mechanical paw sensitivity.
Thermal paw withdrawal latencies (PWLs) were carried out using a thermal plantar analgesia instrument (Ugo Basile, Varese, Italy). The infrared intensity was adjusted to make an average PWL of 10–13 seconds in normal rats, and the cut-off time was set at 15 seconds in advance to prevent tissue damage. Rats were placed into the glass-floored testing cages for 30 minutes to acclimatize before the experiment. The infrared source was placed directly beneath the mid-plantar surface of the hind paw. The heat stimulation was repeated at least 3 times with an interval of about 10 minutes. The mean of the three latencies was used as the PWL. The tests and the data analyzer were double-blinded.
One hundred U/vial BTX-A (catalog No. 20,190,425; Heng-Li, Lanzhou, China) was reconstituted with 1 mL 0.9% saline solution (concentration of 0.1 U BTX-A/μL) laying in 4°C and applied via the intraplantar (i.pl.) route into the ipsilateral hind paw. BTX-A was injected in a volume of 25 μL with a 30-μL microsyringe on the 7th day after CCI modeling. The control group was administered with an equal volume of 0.9% saline solution. No injection was made into the right hind paw. Colchicine (COL) was dissolved in 0.9% saline stored at 4°C, and administered at a concentration 10 mmol/L. The COL solution was used to disrupt axoplasmic transport of the sciatic nerves, and the method of application was performed according to previously standardized protocols [14].
The axoplasmic transport of the sciatic nerves was blocked by COL solution on the 7th day after CCI surgery according to the method described previously [14]. The rats were treated with COL only in the last part of this experiment. Rats were anaesthetized with chloral hydrate. Then the left sciatic nerve, with a 7–8 mm length, was exposed by blunt dissection and was carefully rid of the surrounding connective tissue. The nerve was wrapped with a slice of absorbable gelation sponge (5 mm × 5 mm × 10 mm) saturated in the COL, and a strip of parafilm (6 mm × 20 mm) was placed under the nerve in advance to prevent leakage of the agent onto the surrounding tissue. The objects around the nerve were removed after 15 minutes, with the nerve rinsed with saline. The wound was sewed up using 4-0 surgical sutures. The same surgical procedure was followed except that the nerve was exposed to saline alone.
Rats were anaesthetized with chloral hydrate. The left L4-6 spinal cord, L4-6 DRG, proximal sciatic nerve, and plantar skin was removed and fully ground in radio immunoprecipitation assay lysis buffer according to the manufacture’s instruction (Beyotime, Nantong, China). Total protein (30 ug) from each sample was denatured by boiling in water at 99°C before loading it onto a 10% sodium dodecyl sulfate polyacrylamide gel. Electrophoresis of each gel was administered at 200 mA constant current for 90–120 minutes so that the proteins were subsequently electro-transferred to polyvinylidene fluoride membranes. After being blocked with 5% non-fat milk for 2 hours and incubated with rabbit anti-CXCL13 (1:1,000; Abcam, Cambridge, UK), mouse anti-CXCR5 (1:200; Santa Cruz Biotechnology, Inc., Dallas, TX), rabbit anti-GAT-1 (1:1,000; Gene Tex, Irvine, CA), rabbit anti-GAPDH (1:1,000; Hangzhou Xianzhi, Hangzhou, China) overnight at 4°C, the membranes were incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit immunoglobulin G (Ig G) (1:5,000, Boster, Wuhan, China), or HRP-conjugated goat anti-mouse anti-rabbit Ig G (1:5,000, Boster) for 2 hours. Proteins were detected by an enhanced chemiluminescence detection system (Beyotime) and visualized by exposure to Kodak film. The blot density analysis was quantified by densitometric scanning. The GAPDH was used as the loading control for the proteins in this experiment.
Data are shown as mean ± standard error of the mean. The GraphPad Prism software 7.0 (GraphPad Software, San Diego, CA) was used for statistical analysis. One-way analysis of variance (ANOVA) was followed by post hoc Tukey’s honestly significant difference test, or an unpaired Student’s t-test was used to analyze western blot data. The behavioral data was analyzed using two-way ANOVA with Bonferroni’s multiple comparison test. Differences were considered statistically significant at P < 0.05.
The PWTs and PWLs of rats at 1, 3, 5, 7, 10, 14, and 21 days after CCI surgery were carried out to determine the success of the CCI model. As a result, the PWTs and PWLs of ipsilateral hind paws were decreased significantly at the 3rd day after CCI surgery, and maintained from days 7 to 21 (P < 0.001) (Fig. 1A, C). The PWTs and PWLs of the contralateral hind paws showed no significant difference between two groups (Fig. 1B, D).
Then, the relative levels of CXCL13, CXCR5, and GAT-1 in the L4-6 spinal cord, L4-6 DRG, sciatic nerve, and plantar skin of CCI rats at 0 (sham), 3, 7, 10, 14, and 21 days were measured by western blot. The expression of CXCL13, CXCR5, and GAT-1 showed a dramatic increase in the L4-6 spinal cord, L4-6 DRG, sciatic nerve, and plantar skin at 7, 10, 14, and 21 days after CCI surgery compared to those in sham rats (P < 0.001) (Fig. 2).
To investigate the effects and characteristics of BTX-A, the CCI rats were administered BTX-A at the doses of 4, 7, and 10 U/kg (n = 6–8 in each group) through i.pl. at the 7th day after CCI surgery. The CCI + normal saline (NS) group and sham group (n = 6–8 in each group) were given the same volume of normal saline. The results showed that the BTX-A of three doses all significantly relieved the mechanical and thermal hypersensitivity to some degree compared to the CCI + NS group, and the allodynia effect of the CCI + 10 U/kg BTX-A group showed significant differences compared to the CCI + 7 U/kg BTX-A group and the CCI + 4 U/kg BTX-A group (P < 0.001) (Fig. 3A, C). It’s obviously that the higher dose group had more significant change than the lower dose group. There were significant differences in the data of the contralateral side in each group (Fig. 3B, D).
Western blots were used to detect the expression of CXCL13, CXCR5, and GAT-1 at the 21st day after CCI surgery. BTX-A decreased the overexpression of CXCL13, CXCR5, and GAT-1 induced by CCI surgery in the spinal cord, DRG, sciatic nerve, and plantar skin, and the higher dose (7 and 10 U/kg) group had more reduction than the lower dose group (4 U/kg) (P < 0.001) (Fig. 4). These data declared that an i.pl. injection of BTX-A can change the expression of these proteins not only in the plantar skin, but also in the sciatic nerve, DRG, and spinal cord, and that the analgesic effect of BTX-A is dose-dependent in manner.
Both of the CCI group and the sham group were given 10 U/kg BTX-A or saline through plantar injection on the 7th day after surgery. The results showed that there was no significant difference about PWTs between the sham + 10 U/kg BTX-A and sham + NS groups (Fig. 5A). The PWTs of the CCI + 10 U/kg BTX-A group showed a significant difference compared with the CCI + NS group (P < 0.001) (Fig. 5A), and the PWTs of the contralateral hind paws indicated no significant between-group difference (Fig. 5B).
Western blots were used to detected to levels of CXCL13, CXCR5, and GAT-1 in spinal cord, DRG, sciatic nerve, and plantar skin at the 10th, 14th, and 21st day. Ten U/kg BTX-A decreased the overexpression of CXCL13 and CXCR5 proteins in spinal cord, DRG, sciatic nerve, and plantar skin significantly at the 10th, 14th, and 21st day after CCI surgery (P < 0.001) (Figs. 6D, 7). The expression of GAT-1 in spinal cord, DRG and sciatic nerve was decreased significantly at the 14th and 21st day after CCI surgery (P < 0.001) (Figs. 6B, C, 7). And in the mass, the levels of CXCL13, CXCR5, and GAT-1 showed a downward trend among the 10th, 14th, and 21st day as a whole in the CCI + 10 U/kg BTX-A group.
To indicate the role of axon transport in BTX-A analgesia and neuropathic pain, the sciatic nerve was treated by 10 mmol/L COL at 7th day after surgery to block the axonal transport. Meanwhile, the 10 U/kg BTX-A was administered to rats through i.pl. on the 7th day. The PWTs of the CCI + COL + NS group and CCI + COL + 10 U/kg BTX-A group were significantly relieved at the 10th day, and steadily maintained at 15 g above from days 10 to 21 (P < 0.001) (Fig. 8A). In the present experiment, if the PWT was greater than 15 g, it was recorded as 15 g. As a result, the line charts of the CCI + COL + NS group and CCI + COL + 10 U/kg BTX-A group overlapped at the 10th, 14th, and 21st day after CCI surgery. The mechanical pain threshold on the contralateral hind paw did not show a significant difference between groups (Fig. 8B).
To explore the role of axonal transport on neuropathic pain and the analgesic effect of BTX-A, the sciatic nerves of rats were infiltrated by saline or 10 mmol/L COL solution and the ipsilateral plantar hind paws were injected with saline or 10 U/kg BTX-A on the 7th day after CCI surgery. The rats were anesthetized and their spinal cord, DRG, sciatic nerve, and the plantar surface of the ipsilateral hind paw were removed for western blot tests on the 21st day after CCI surgery. The results showed that the expression of CXCL13, CXCR5, and GAT-1 in the plantar surface of the hind paw, sciatic nerve, DRG, and spinal cord in the CCI + COL + NS group was decreased significantly compared with the CCI + NS + NS group (P < 0.001) (Fig. 9). And there was no significant difference between the CCI + COL + BTX-A group and the CCI + COL + NS group. The above results showed that the inhibitory effect of BTX-A on the expression of CXCL13, CXCR5, and GAT-1 in the spinal cord and DRG disappeared. After the axonal transport of the sciatic nerve was blocked, the overexpression of CXCL13, CXCR5, and GAT-1 in the spinal cord and DRG after CCI surgery was decreased. It showed that blocking the axonal transport of the sciatic nerve may also block the pain-causing molecules transport to the spinal cord and DRG.
In this study, BTX-A injected through the plantar surface of the hind paw relieved the pain behavior and reversed the overexpression of CXCL13/CXCR5 and GAT-1 in the spinal cord, DRG, sciatic nerve, and plantar skin in CCI rats. After 10 mmol/L COL blocked the axon transport of the sciatic nerve, the inhibitory effect of BTX-A disappeared, and the levels of CXCL13/CXCR5 and GAT-1 in the spinal cord and DRG were decreased in CCI rats.
The molecular mechanisms of BTX-A involve inflammatory factors. Studies have reported that BTX-A prevented the release of neurotransmitters such as acetylcholine and glutamate [15], decreased the overexpression of the pronociceptive proteins interleukin (IL)-18, IL-6, and IL-1β and increased the levels of antinociceptive proteins IL-10 and IL-1RA in spinal cord and DRG in CCI rats [16]. In the authors’ studies, BTX-A suppressed the overexpression of CXCL13, CXCR5, and GAT-1 in the planter skin, sciatic nerve, DRG and spinal cord in CCI rats to relieve mechanical pain and can be characterized as acting in a dose-dependent manner.
The analgesic effect of BTX-A depends on axonal transport. In the authors’ experiment, BTX-A produces an analgesic effect by inhibiting the high expression of CXCL13 and GAT-1 in CCI rats. However, after COL was used to block axonal transport, the inhibitory effect of botulinum toxin on the expression of CXCL13 and GAT-1 disappeared. These results suggested that BTX-A plays an analgesic role through axonal transport, which is consistent with previous studies [10].
The axonal transport plays an important role in neuropathic pain. Curtis et al. [17] investigated the retrograde axonal transport of neurotrophins (nerve growth factor, brain-derived neurotrophic factor, et al.) from the sciatic nerve to the DRG and spinal neurons in normal rats and after nerve injury [17]. And studies also showed that tumor necrosis factor-alpha and protein kinase G transported from the injury site to the DRG [18,19], and CXCL12 and CXCR4 were transported from nerve cell bodies to the spinal dorsal horn to promote the development of neuropathic pain [20]. In the present study, when the sciatic nerve was treated with COL, the levels of CXCL13 and GAT-1 in the DRG and spinal cord were decreased (Fig. 9). The authors speculate that the decrease of CXCL13 and GAT-1 in the spinal cord and DRG may be due to the inhibitory effect of COL on pain signals and related pain mediators, but there is also a new possibility that there may be mutual transmission of molecules such as CXCL13 and GAT-1 along the nerve fibers between peripheral nociceptors and the DRG or spinal cord. COL can block the transport of CXCL13 and GAT-1 from a sciatic nerve injury to the DRG and spinal cord by blocking the axonal transport of the sciatic nerve.
Chemokine is involved in axonal transport. The major role of chemokines is to act as a chemoattractant to guide the migration of cells [21–23]. Cells are attracted to move through the gradient towards the higher concentration of chemokine [24]. It can be seen that chemokine needs to be transported to form a concentration gradient from high to low. The injury site of the CCI model in the present experiment is the sciatic nerve. According to the chemotactic characteristics of chemokines, the CXCL13 produced by the sciatic nerve can move to the distal end of the injury site to form a concentration gradient to attract cells to the injury site. The results further support the authors’ speculation that CXCL13 and GAT-1 may transfer from the injured site to DRG and spinal cord through axonal transport.
It is worth mentioning that after the sciatic nerve was treated with COL, the PWT of the hind paw of CCI rats was significantly increased, and remained above 15 g from 10 days to 21 days after the operation. It is obvious that such an analgesic effect can’t be achieved by blocking the axonal molecular transport with COL. This may be caused by other mechanisms of COL. The authors speculate that most of the electrical signals in the injured part of the sciatic nerve can’t be transmitted to the DRG or spinal cord after COL treatment [25], which makes the plantar pain sensation caused by CCI surgery to be dull or disappear.
In conclusion, BTX-A produced anti-allodynic and anti-hyperalgesic effects, and inhibited the overexpression of CXCL13, CXCR5, and GAT-1 in CCI rats through axon transport. CXCL13 may be transported from the injury site to spine or DRG through axonal transport. Axon molecular transport may be a target to enhance pain management in neuropathic pain.
Notes
DATA AVAILABILITY
Data files are available from Harvard Dataverse: https://doi.org/10.7910/DVN/MCGNJT.
REFERENCES
1. Baba M, Takatsuna H, Matsui N, Ohwada S. 2020; Mirogabalin in Japanese patients with renal impairment and pain associated with diabetic peripheral neuropathy or post-herpetic neuralgia: a phase III, open-label, 14-week study. J Pain Res. 13:1811–21. DOI: 10.2147/JPR.S255345. PMID: 32765056. PMCID: PMC7381826. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85088415306&origin=inward.
2. Rodríguez MJ, Díaz S, Vera-Llonch M, Dukes E, Rejas J. 2007; Cost-effectiveness analysis of pregabalin versus gabapentin in the management of neuropathic pain due to diabetic polyneuropathy or post-herpetic neuralgia. Curr Med Res Opin. 23:2585–96. DOI: 10.1185/030079907X233151. PMID: 17875242. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=35648987172&origin=inward.

3. Gerken JD, Fritzsche T, Denke C, Schäfer M, Tafelski S. 2020; Retrospective study on ganglionic and nerve block series as therapeutic option for chronic pain patients with refractory neuropathic pain. Pain Res Manag. 2020:6042941. DOI: 10.1155/2020/6042941. PMID: 32774567. PMCID: PMC7399767. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85089321936&origin=inward.

4. Liu B, Yang Y, Zhang Z, Wang H, Fan B, Sima L. 2020; Clinical study of spinal cord stimulation and pulsed radiofrequency for management of herpes zoster-related pain persisting beyond acute phase in elderly patients. Pain Physician. 23:263–70. PMID: 32517392. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85085974146&origin=inward.
5. Li Z, Guo Y, Ren X, Rong L, Huang M, Cao J, et al. 2019; HDAC2, but not HDAC1, regulates Kv1.2 expression to mediate neuropathic pain in CCI rats. Neuroscience. 408:339–48. DOI: 10.1016/j.neuroscience.2019.03.033. PMID: 31022463. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85065047403&origin=inward.

6. Tal M, Eliav E. 1996; Abnormal discharge originates at the site of nerve injury in experimental constriction neuropathy (CCI) in the rat. Pain. 64:511–8. DOI: 10.1016/0304-3959(95)00175-1. PMID: 8783316. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0029893712&origin=inward.

7. Zhang JM, Li H, Munir MA. 2004; Decreasing sympathetic sprouting in pathologic sensory ganglia: a new mechanism for treating neuropathic pain using lidocaine. Pain. 109:143–9. DOI: 10.1016/j.pain.2004.01.033. PMID: 15082136. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=1842786067&origin=inward.

8. Serra J, Bostock H, Solà R, Aleu J, García E, Cokic B, et al. 2012; Microneurographic identification of spontaneous activity in C-nociceptors in neuropathic pain states in humans and rats. Pain. 153:42–55. DOI: 10.1016/j.pain.2011.08.015. PMID: 21993185. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84155162846&origin=inward.

9. Li L, Shao J, Wang J, Liu Y, Zhang Y, Zhang M, et al. 2019; MiR-30b-5p attenuates oxaliplatin-induced peripheral neuropathic pain through the voltage-gated sodium channel Nav1.6 in rats. Neuropharmacology. 153:111–20. DOI: 10.1016/j.neuropharm.2019.04.024. PMID: 31054938. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85065535209&origin=inward.

10. Matak I, Riederer P, Lacković Z. 2012; Botulinum toxin's axonal transport from periphery to the spinal cord. Neurochem Int. 61:236–9. DOI: 10.1016/j.neuint.2012.05.001. PMID: 22580329. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84863449492&origin=inward.

11. Bu HL, Xia YZ, Liu PM, Guo HM, Yuan C, Fan XC, et al. 2019; The roles of chemokine CXCL13 in the development of bone cancer pain and the regulation of morphine analgesia in rats. Neuroscience. 406:62–72. DOI: 10.1016/j.neuroscience.2019.02.025. PMID: 30826523. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85062966103&origin=inward.

12. Ford A, Castonguay A, Cottet M, Little JW, Chen Z, Symons-Liguori AM, et al. 2015; Engagement of the GABA to KCC2 signaling pathway contributes to the analgesic effects of A3AR agonists in neuropathic pain. J Neurosci. 35:6057–67. Erratum in: J Neurosci 2015; 35: 8971. DOI: 10.1523/JNEUROSCI.4495-14.2015. PMID: 25878279. PMCID: PMC4397603. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84929243393&origin=inward.
13. Bennett GJ, Xie YK. 1988; A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 33:87–107. DOI: 10.1016/0304-3959(88)90209-6. PMID: 2837713. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0023895875&origin=inward.

14. Dilley A, Bove GM. 2008; Disruption of axoplasmic transport induces mechanical sensitivity in intact rat C-fibre nociceptor axons. J Physiol. 586:593–604. DOI: 10.1113/jphysiol.2007.144105. PMID: 18006580. PMCID: PMC2375581. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=38149084163&origin=inward.

15. Akaike N, Shin MC, Wakita M, Torii Y, Harakawa T, Ginnaga A, et al. 2013; Transsynaptic inhibition of spinal transmission by A2 botulinum toxin. J Physiol. 591:1031–43. DOI: 10.1113/jphysiol.2012.242131. PMID: 23109108. PMCID: PMC3591713. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84873738735&origin=inward.

16. Zychowska M, Rojewska E, Makuch W, Luvisetto S, Pavone F, Marinelli S, et al. 2016; Participation of pro- and anti-nociceptive interleukins in botulinum toxin A-induced analgesia in a rat model of neuropathic pain. Eur J Pharmacol. 791:377–88. DOI: 10.1016/j.ejphar.2016.09.019. PMID: 27619001. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84987918398&origin=inward.

17. Curtis R, Tonra JR, Stark JL, Adryan KM, Park JS, Cliffer KD, et al. 1998; Neuronal injury increases retrograde axonal transport of the neurotrophins to spinal sensory neurons and motor neurons via multiple receptor mechanisms. Mol Cell Neurosci. 12:105–18. DOI: 10.1006/mcne.1998.0704. PMID: 9790733. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0031727821&origin=inward.

18. Myers RR, Shubayev VI. 2011; The ology of neuropathy: an integrative review of the role of neuroinflammation and TNF-α axonal transport in neuropathic pain. J Peripher Nerv Syst. 16:277–86. DOI: 10.1111/j.1529-8027.2011.00362.x. PMID: 22176142. PMCID: PMC4444223. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=83755184177&origin=inward.

19. Sung YJ, Chiu DTW, Ambron RT. 2006; Activation and retrograde transport of protein kinase G in rat nociceptive neurons after nerve injury and inflammation. Neuroscience. 141:697–709. DOI: 10.1016/j.neuroscience.2006.04.033. PMID: 16730916. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33746001390&origin=inward.

20. Reaux-Le Goazigo A, Rivat C, Kitabgi P, Pohl M, Melik Parsadaniantz S. 2012; Cellular and subcellular localization of CXCL12 and CXCR4 in rat nociceptive structures: physiological relevance. Eur J Neurosci. 36:2619–31. DOI: 10.1111/j.1460-9568.2012.08179.x. PMID: 22694179. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84865865940&origin=inward.

21. Laganà M, Schlecht-Louf G, Bachelerie F. 2021; The G protein-coupled receptor kinases (GRKs) in chemokine receptor-mediated immune cell migration: from molecular cues to physiopathology. Cells. 10:75. DOI: 10.3390/cells10010075. PMID: 33466410. PMCID: PMC7824814. PMID: 5c5a4905ae9a4538882747e916612998. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85100226063&origin=inward.

22. Schioppa T, Sozio F, Barbazza I, Scutera S, Bosisio D, Sozzani S, et al. 2020; Molecular basis for CCRL2 regulation of leukocyte migration. Front Cell Dev Biol. 8:615031. DOI: 10.3389/fcell.2020.615031. PMID: 33363177. PMCID: PMC7758318. PMID: 99ae512bd2604cdbae7654e1378ccbc7. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85098201685&origin=inward.

23. Jiang BC, Liu T, Gao YJ. 2020; Chemokines in chronic pain: cellular and molecular mechanisms and therapeutic potential. Pharmacol Ther. 212:107581. DOI: 10.1016/j.pharmthera.2020.107581. PMID: 32450191. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85085585010&origin=inward.

24. Lau S, Feitzinger A, Venkiteswaran G, Wang J, Lewellis SW, Koplinski CA, et al. 2020; A negative-feedback loop maintains optimal chemokine concentrations for directional cell migration. Nat Cell Biol. 22:266–73. DOI: 10.1038/s41556-020-0465-4. PMID: 32042179. PMCID: PMC7809593. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85079456516&origin=inward.

25. Proske U, Luff AR. 1998; Mechanical sensitivity of muscle afferents in a nerve treated with colchicine. Exp Brain Res. 119:391–8. DOI: 10.1007/s002210050354. PMID: 9551839. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0031932974&origin=inward.

Fig. 1
In chronic constriction injury (CCI) rats, the paw withdrawal thresholds (PWTs) and paw withdrawal latencies (PWLs) in the ipsilateral were decreased from day 3 after modeling and reached a stable level from day 7 (A, C). The PWTs and PWLs in the contralateral side in each group had no significant changes in time points (B, D). The error bar means mean ± standard deviation. aP < 0.05 vs. sham; Two-way ANOVA; n = 11 rats in each group.
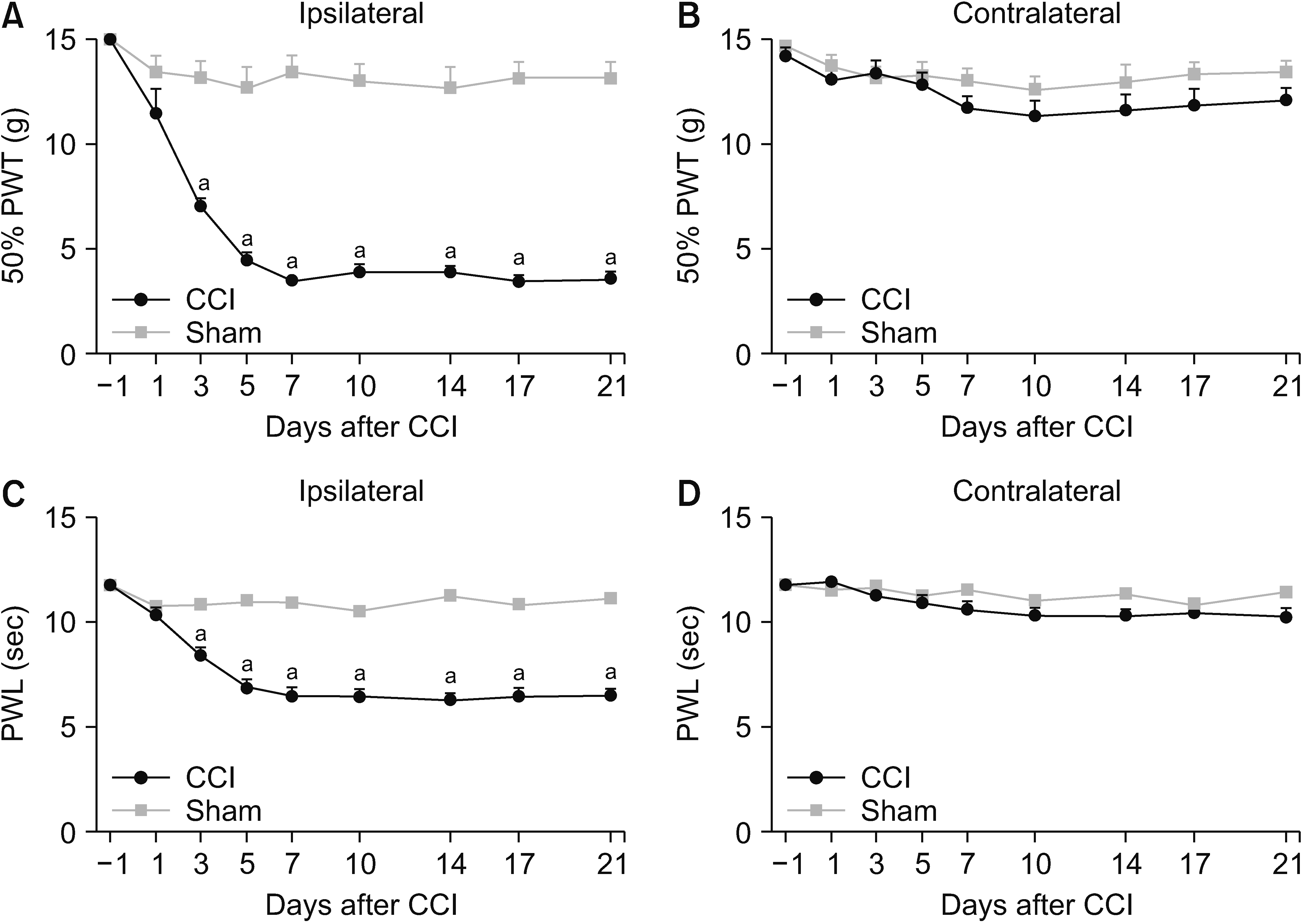
Fig. 2
Chronic constriction injury (CCI) of the sciatic nerve upregulated the expression of CXCL13, CXCR5, and GAT-1 from day 3 or 7 in the spinal cord, DRG, sciatic nerve, and plantar skin by western blot. (A, E, I, M) The expression of CXCL13, CXCR5, and GAT-1 in the spinal cord. (B, F, J, N) The expression of these proteins in the DRG. (C, G, K, O) The expression of these proteins in the sciatic nerve. (D, H, L, P) The expression of these proteins in the plantar skin. The error bar means mean ± standard deviation. CXCL13: chemokine ligand 13, CXCR5: C-X-C chemokine receptor type 5, GAT-1: GABA transporter 1, DRG: dorsal root ganglia. aP < 0.05 vs. sham; One-way ANOVA; n = 3 in each group.
Fig. 3
In chronic constriction injury (CCI) rats, the paw withdrawal thresholds (PWTs) and paw withdrawal latencies (PWLs) in the ipsilateral side were decreased from day 3 after modeling. Botulinum toxin type A (BTX-A) (4, 7, 10 U/kg) reversed these decreases from day 10 in a dose-dependent manner (A, C). The PWTs and PWLs of the contralateral side in each group exhibited no significant changes in time points (B, D). The error bar means mean ± standard deviation. aP < 0.05 vs. CCI + normal saline (NS) group, Two-way ANOVA, n = 6 in each group; bP < 0.05 vs. CCI + 4 U/kg BTX-A group, Two-way ANOVA, n = 6 in each group; cP < 0.05 vs. CCI + 7 U/kg BTX-A group, Two-way ANOVA, n = 6 in each group.
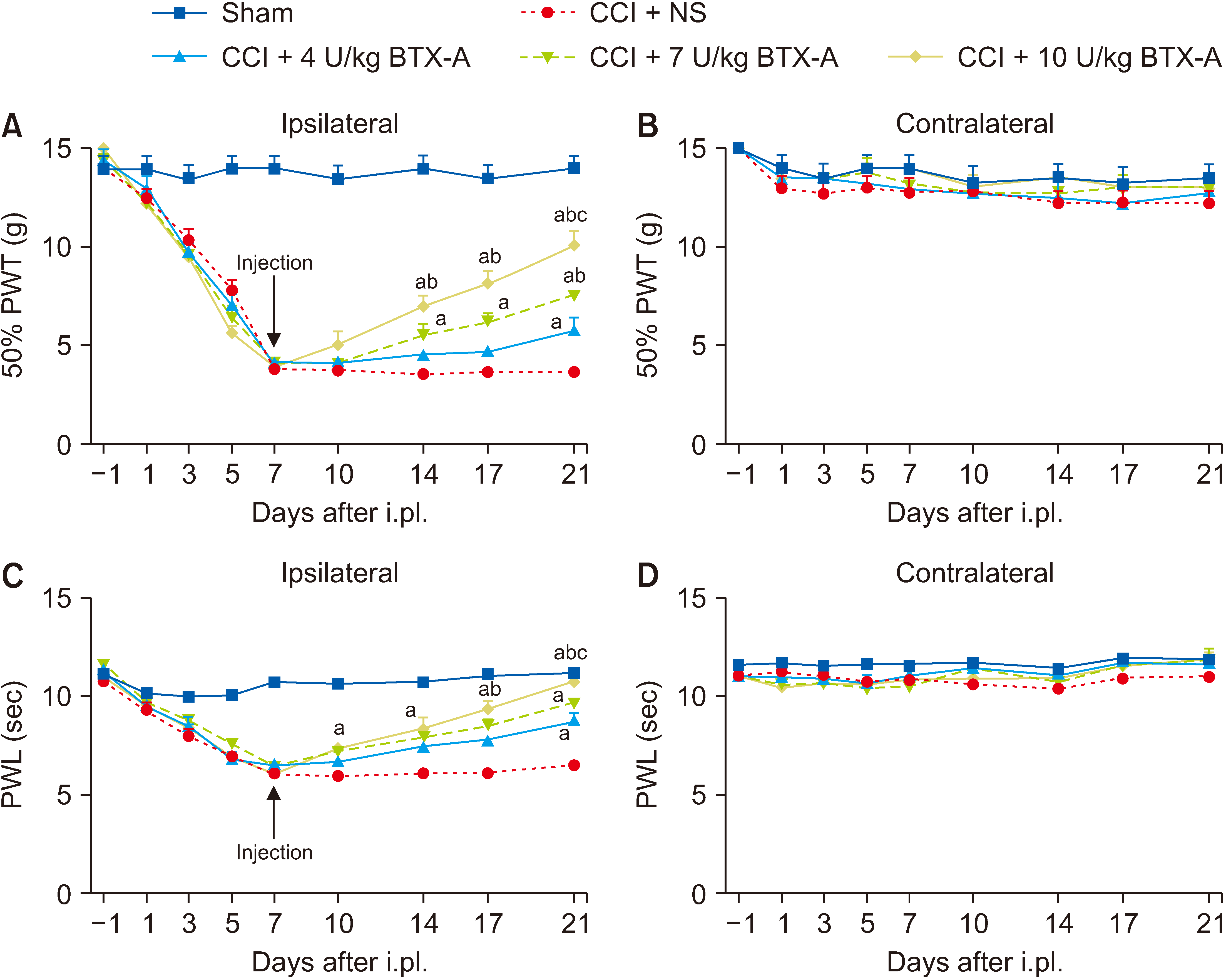
Fig. 4
The botulinum toxin type A (BTX-A) inhibited the overexpression of CXCL13, CXCR5, and GAT-1 in chronic constriction injury (CCI) rats, and the effect of BTX-A is dose-dependent. The BTX-A reversed the increased expression of CXCL13, CXCR5, and GAT-1 induced by CCI in the spinal cord tested by western blot (A, E, I, M), DRG (B, F, J, N), sciatic nerve (C, G, K, O), and plantar skin (D, H, L, P). CXCL13: chemokine ligand 13, CXCR5: C-X-C chemokine receptor type 5, GAT-1: GABA transporter 1, DRG: dorsal root ganglia. The error bar means mean ± standard deviation. aP < 0.05 vs. sham group, bP < 0.05 vs. CCI + normal saline group, cP < 0.05 vs. CCI + 4 U/kg BTX-A group, dP < 0.05 vs. CCI + 10 U/kg BTX-A group; One-way ANOVA; n = 3 in each group.
Fig. 5
Botulinum toxin type A (BTX-A) injection reversed the decrease of paw withdrawal thresholds (PWTs) in the ipsilateral hind paw of chronic constriction injury (CCI) rats from day 10 after modeling (A). However, the PWTs in the contralateral side had no significant changes in each group (B). The error bar means mean ± standard deviation. aP < 0.05 vs. CCI + normal saline (NS) group, Two-way ANOVA, n = 6 in each group.
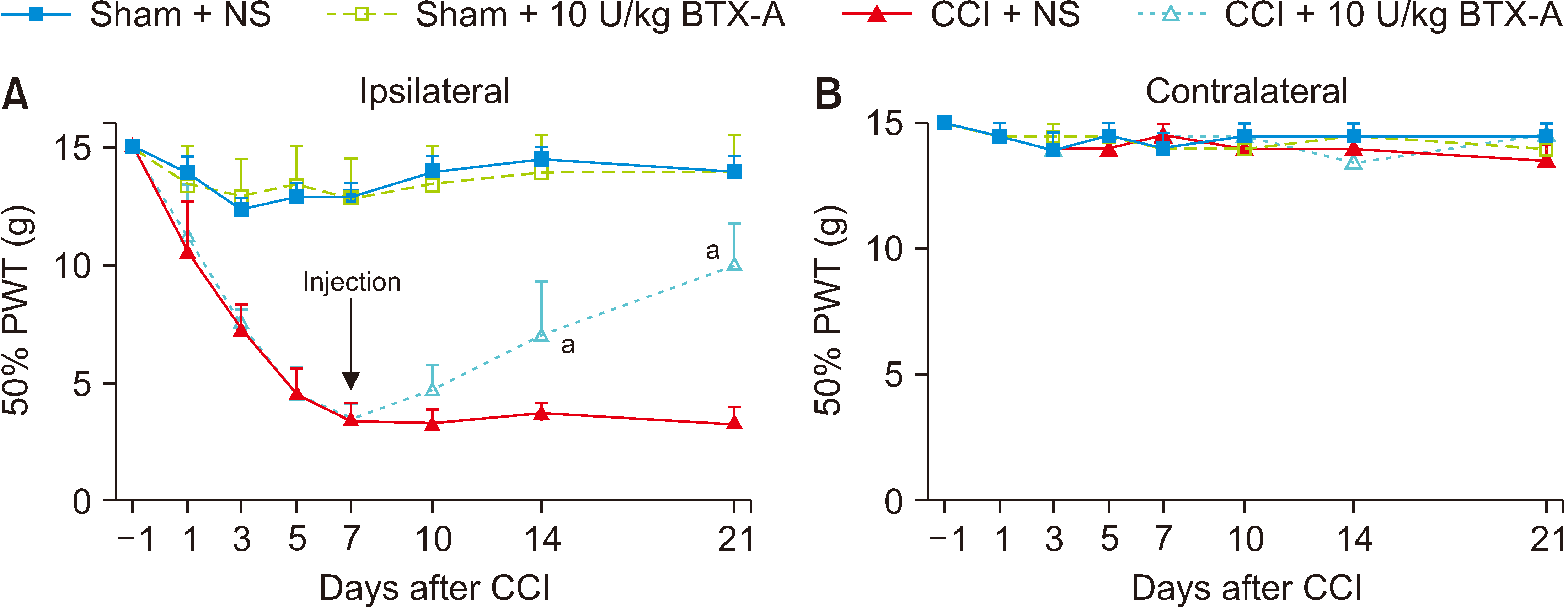
Fig. 6
Western blot analysis showing changes in CXCL13, CXCR5, and GAT-1 levels of the spinal cord at 10, 14, and 21 days after surgery. Treatment with botulinum toxin type A (BTX-A) prevented the increase in CXCL13, CXCR5, and GAT-1 proteins as graphed in A, B, C, and D. The error bar means mean ± standard deviation. CCI: chronic constriction injury, CXCL13: chemokine ligand 13, CXCR5: C-X-C chemokine receptor type 5, GAT-1: GABA transporter 1. aP < 0.05 vs. CCI + normal saline (NS) group, One-way ANOVA, n = 3 in each group.
Fig. 7
(A–C) Western blot data showing that the increased expression of CXCL13, CXCR5, and GAT-1 proteins after chronic constriction injury (CCI) surgery in the DRG, sciatic nerve, and plantar skin was reversed by botulinum toxin type A (BTX-A). CXCL13: chemokine ligand 13, CXCR5: C-X-C chemokine receptor type 5, GAT-1: GABA transporter 1, DRG: dorsal root ganglia. The error bar means mean ± standard deviation. aP < 0.05 vs. CCI + normal saline (NS) group, One-way ANOVA, n = 3 in each group.
Fig. 8
The mechanical hypersensitivity after chronic constriction injury (CCI) surgery was attenuated after the sciatic nerve was treated with 10 mmol/L colchicine (COL) (A). The paw withdrawal thresholds (PWTs) in the contralateral hind paw of each group had no significant change at time points (B). The dose of botulinum toxin type A (BTX-A) which was used was 10 U/kg. The error bar means mean ± standard deviation. aP < 0.05 vs. CCI + normal saline (NS) + NS group, Two-way ANOVA, n = 6 in each group. bP < 0.05 vs. CCI + NS + 10 U/kg BTX-A group, Two-way ANOVA, with n = 6 in each group.
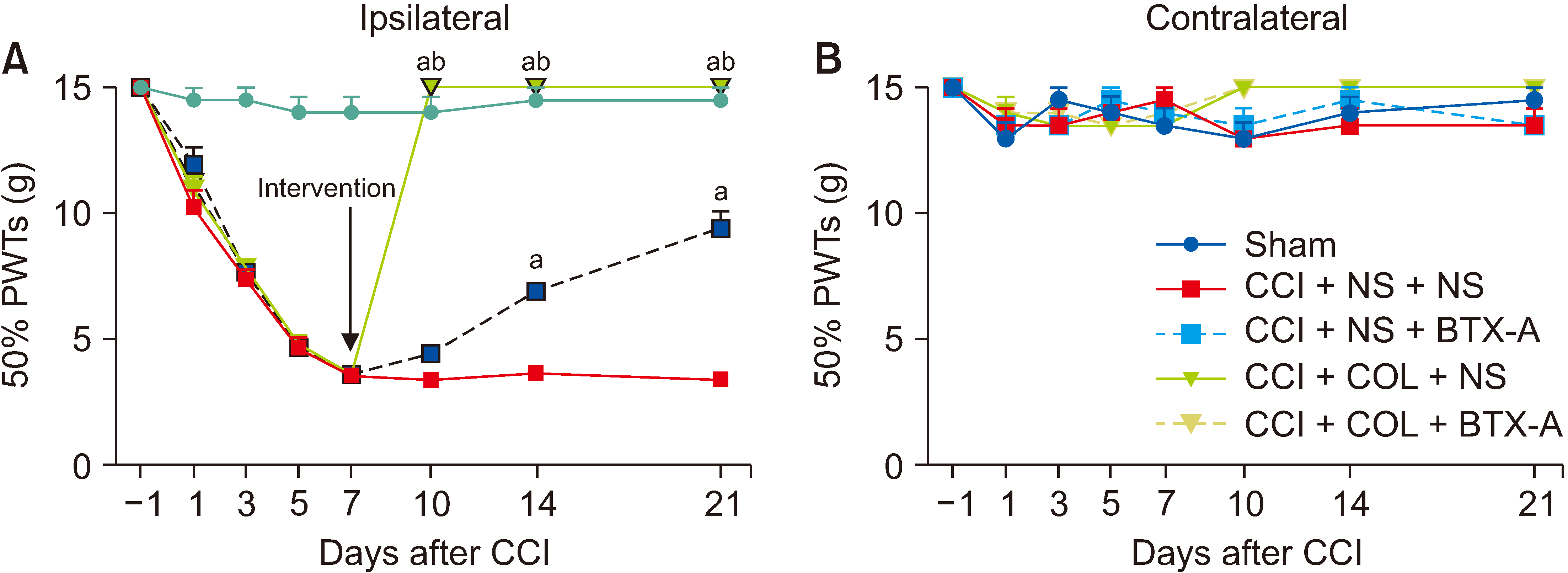
Fig. 9
Rats were treated with colchicine (COL, 10 mmol/L) in the sciatic nerve, and (or) botulinum toxin type A (BTX-A, 10 U/kg) injected to the plantar surface of the hind paw. The levels of CXCL13, CXCR5, and GAT-1 were increased in the spinal cord, DRG, sciatic nerve and hind paw of chronic constriction injury (CCI) rats. Both COL and BTX-A attenuated these increases. But in the spinal cord (A, E, I, M) and DRG (B, F, J, N), combination of COL and BTX-A could partly reversed BTX-A-induced CXCL13, CXCR5, and GAT-1 inhibition. The effect of combination of COL and BTX-A on the BTX-A-induced inhibition of GAT-1 in sciatic nerve (O) and CXCR5 in hind paw (L) was not significant. The trends of the expression of CXCL13 in sciatic nerve (C, G) and hind paw (D, H), CXCR5 in sciatic nerve (K), and GAT-1 in hind paw (P) were similar with those in spinal cord and DRG. These suggest that blocking axonal transport by COL can reverse BTX-A-induced inhibition neuro-inflammation. The error bar means mean ± standard deviation. CXCL13: chemokine ligand 13, CXCR5: C-X-C chemokine receptor type 5, GAT-1: GABA transporter 1, DRG: dorsal root ganglia, NS: normal saline. aP < 0.05 vs. the sham group, bP < 0.05 vs. the CCI-NS-NS group, cP < 0.05 vs. CCI-COL-NS group, One-way ANOVA, n = 3 in each group.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download