INTRODUCTION
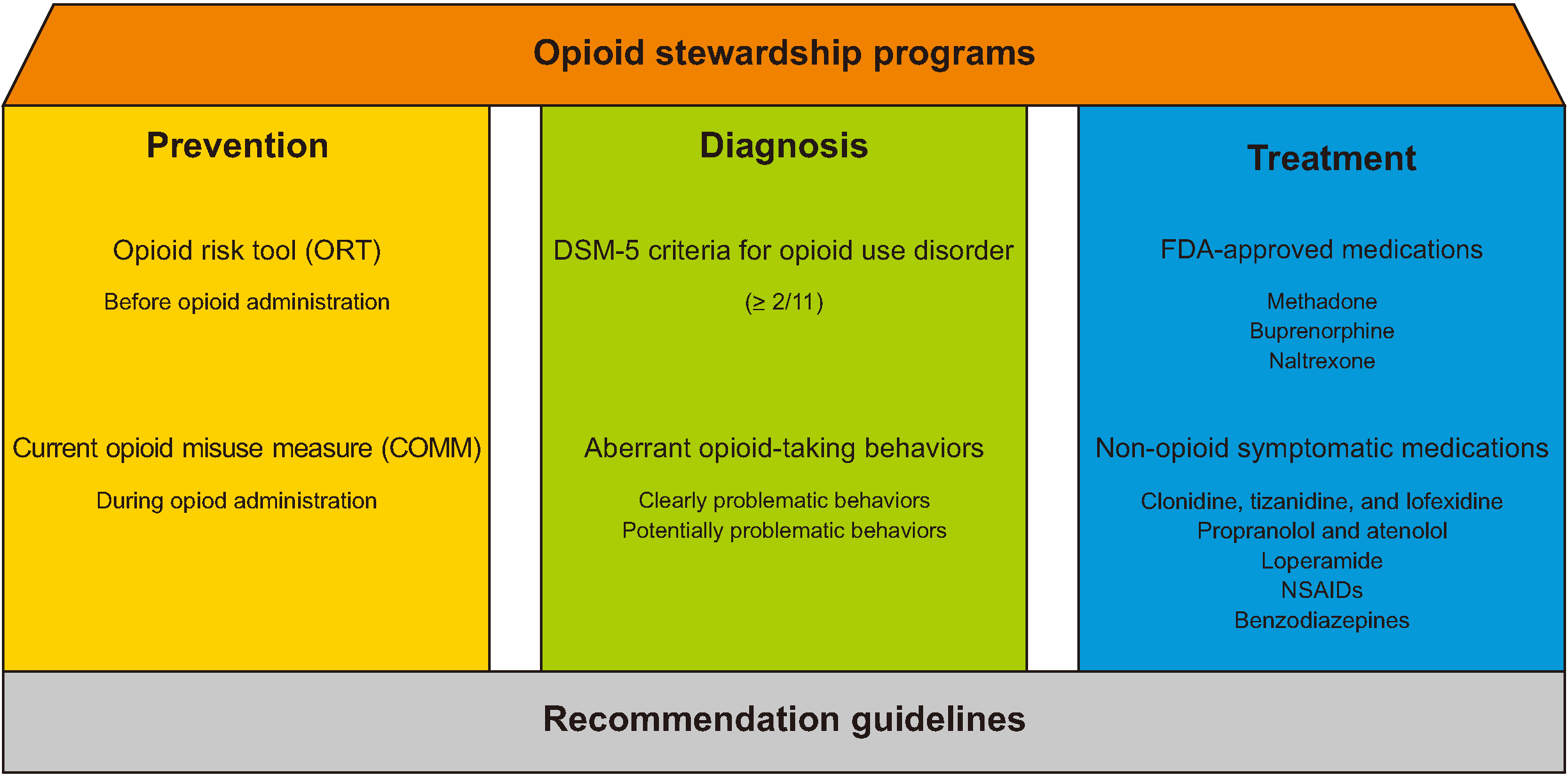 | Fig. 1Schematic illustration of prevention, diagnosis, and treatment of opioid use disorders (OUD). Under the supervision of opioid stewardship programs (roof), prevention, diagnosis and treatment of opioid use disorder (3 pillars) should be performed, based on the various recommendation guidelines (foundation). Prevention of OUD is recommended to use the screening tests, such as opioid risk tool (ORT) before opioid administration and current opioid misuse measure (COMM). Diagnosis of OUD is performed by the Diagnosis and Statistical Manual of Mental Disorders, fifth edition (DSM-5). OUD is suspected if patients have 2 or more among 11 items. For busy clinicians, aberrant opioid-taking behaviors, composed of clearly and potentially problematic behaviors, can be used for suspicion of OUD in a clinical field. Treatment of OUD consists of 3 the U.S. Food and Drug Administration (FDA)-approved medications, including methadone, buprenorphine, and naltrexone and non-opioid symptomatic medications for the treatment of opioid withdrawal syndrome, such as α2 agonists, β-blockers, antidiarrheals, antiemetics, non-steroidal anti-inflammatory drugs (NSAIDs), and benzodiazepines. These methods for prevention, diagnosis, and treatment are based on the 6 recommendation guidelines from various societies and associations, under the supervision of opioid stewardship programs. |
MAIN BODY
1. OSPs
2. Essential terms related to OUD
Table 1
| Terms | Definition | References | ||
|---|---|---|---|---|
| Opioid dependence | A cluster of cognitive, behavioral, and physiological features (≥ 3/6) | ICD-10 [18] | ||
| ① A strong desire or sense of compulsion to take opioid | ||||
| ② Difficulties in controlling opioid use | ||||
| ③ A psychological withdrawal state | ||||
| ④ Tolerance | ||||
| ⑤ Progressive neglect of alternative pleasure or interests because of opioid use | ||||
| ⑥ Persisting with opioid use of despite clear evidence of overtly harmful consequences | ||||
| Opioid use disorder (OUD) | Opioid use and the repeated occurrence with 1 year (≥ 2/11), 2–3: mild, 4–5: moderate, and ≥ 6: severe | DSM-5 [20] | ||
| ① Continued use despite worsening physical or psychological health | ||||
| ② Continued use leading to social and interpersonal consequences | ||||
| ③ Decreased social or recreational activities | ||||
| ④ Difficulty fulfilling professional duties at school or work | ||||
| ⑤ Excessive time to obtain opioids, or recover from taking them | ||||
| ⑥ More taken than intended | ||||
| ⑦ Cravings | ||||
| ⑧ Unable to decrease the amount used | ||||
| ⑨ Tolerance | a. A need for markedly increased amounts of opioids to achieve intoxication or desired effect. | |||
| b. A markedly diminished effect with continued use of the same amount of an opioid. | ||||
| ⑩ Use despite physically dangerous settings | ||||
| ⑪ Withdrawal | ||||
| Aberrant opioid-taking behaviors related to OUD | Clearly problematic | Potentially problematic | Brady et al. [21] | |
| ① Selling opioids | ① Hoarding | |||
| ② Forging prescriptions | ② Requesting a certain type of opioid | |||
| ③ Stealing opioids from others | - | |||
| ④ Use by non-prescribed route | - | |||
| ⑤ Doctor shopping | - | |||
| ⑥ Repeated loss of opioids and running out early | ③ A single loss of opioid and running out early | |||
| ⑦ Multiple increases in dosage | ④ A single increase in dosage | |||
| Opioid addiction | A primary chronic neurobiological disease, produced by repeated exposure to an addictive opioid and characterized by loss of control over opioid use (≥ 1/4) | Ballantyne and LaForge [23] | ||
| ① A pronounced craving for the opioid | ||||
| ② Obsessive thinking about the opioid | ||||
| ③ Erosion of inhibitory control over efforts to refrain from opioid use | ||||
| ④ Compulsive opioid taking | ||||
| Opioid pseudoaddiction | An opioid seeking situation due to inadequate pain treatment, relieved by adequate pain management | Brady et al. [21] | ||
| Opioid physical dependence | A state of adaptation that is manifested an opioid specific withdrawal syndrome, produced by abrupt cessation, rapid dose reduction, decreasing blood level, and/or administration of an antagonist | Ballantyne and LaForge [23] | ||
| Opioid tolerance (insensitivity) | Need for increasing dose of opioid to achieve the same effect or diminished response to a opioid with repeated use | Dowell et al. [13] | ||
| Ballantyne and LaForge [23] | ||||
| Opioid withdrawal syndrome (OWS) | A opioid-specific problematic behavioral change, with physiologic and cognitive components, that is due to the cessation of, or reduction in, heavy and prolonged opioid use | DSM-5 [20] | ||
| A group of symptoms of variable clustering and severity occurring on absolute or relative withdrawal of a psychoactive opioid after persistent use of that opioid | ICD-10 [18] | |||
| A collection of characteristic clinical symptoms and signs, which include hypertension, tachycardia, mydriasis, piloerection, lacrimation, rhinorrhea, yawning, insomnia, nausea, vomiting, and diarrhea | Srivastava et al. [24] | |||
| Opioid misuse (non-medical opioid use) | Any use outside of prescription parameters | Brady et al. [21] | ||
| ① Misunderstanding of instructions | Kosten and Baxter [25] | |||
| ② Self-medication for sleep mood, or anxiety regardless of pain | ||||
| ③ Compulsive use driven by OUD | ||||
| Opioid abuse | Use of opioids without a prescription | Brady et al. [21] | ||
| Opioid diversion | The intentional transfer of opioid from authorized to unauthorized possession | Inciardi et al. [26] | ||
| Morphine milligram equivalent per day (MME/d) | An opioid daily dosage’s equivalency to morphine | |||
| Weak opioids | tramadol (0.1), meperidine (0.1), codeine (0.15) | Dowell et al. [13] | ||
| Moderate opioid | tapentadol (0.4) | |||
| Strong opioids | morphine (1), hydrocodone (1), oxycodone (1.5), oxymorphone (3), hydromorphone (4), methadone (1–20: 4, 21–40: 8, 41–60: 10, and 61–80: 12), transdermal fentanyl patch (μg, 2.4) | |||
Methadone shows a different morphine milligram equivalent per day according to its dosage: 1–20 mg/d of methadone is equivalent to 4 mg/d of morphine; 21–40 mg/d of methadone is equivalent to 8 mg/d of morphine; 41–60 mg/d of methadone is equivalent to 10 mg/d of morphine; 61–80 mg/d of methadone is equivalent to 12 mg/d of morphine.
3. Problems in opioid use for pain
1) Problems in opioid use for acute pain
(1) Intraoperative infusion of remifentanil
(2) Patient-controlled analgesia for postoperative pain
2) Problems in opioid use for chronic pain
(1) Problems in opioid use for CNCP
(2) Problems in opioid use for cancer pain
4. Various recommendations related to OUD
1) Twelve recommendations from the U. S. CDC for prescribing opioids for chronic pain in 2016
(1) Determination for initiation or continuation of opioids for chronic pain
① Begin with non-pharmacologic and non-opioid pharmacologic therapy. Opioid therapy should be appropriately added to non-pharmacologic and non-opioid pharmacologic therapy. If benefits for patient’s pain management and physical functions can be expected to outweigh the risks, then opioid therapy should be considered.
② Create realistic opioid treatment goals prior to administration, and give attention to methods for discontinuation when benefits do not outweigh risks.
③ Discuss with patients the benefits and risks before and during the opioid therapy.
(2) Selection, follow-up, and discontinuation of opioids
④ Start from IR rather than ER/long-acting opioids.
⑤ Start from the lowest effective dosage. Use caution if the dosage is reaching 50 MME/d, and avoid a dosage above 90 MME/d.
⑥ Start IR opioids only for 3 days or less and limited to 7 days, for acute pain.
⑦ Reevaluate the benefits/harms or dose escalation for chronic pain within 1 to 4 weeks and at least every 3 months. Consider tapering or discontinuing whenever benefits do not outweigh harms.
(3) Evaluation of risk factors and treatment
⑧ Evaluate risk factors, such as history of opioid overdose, other SUD, higher opioid dosage ≥ 50 MME/d, or concurrent use of benzodiazepine.
⑨ Review the history of controlled substances when starting opioids and periodically at every 3 months, using State Prescription Drug Monitoring Program (PDMP) data.
⑩ Use urine examination for prescribed medications, controlled prescriptions, and illicit drugs at least annually.
⑪ Avoid prescribing concurrent administration of benzodiazepines.
⑫ Offer medication-assisted treatment (MAT) and behavioral therapies for OUD.
2) Ten recommendations from the Canadian guideline for opioid therapy for CNCP in 2017 [62]
① Start with non-opioid medication and non-pharmacologic therapy, rather than opioid therapy in CNCP.
② Avoid opioid therapy in patients with a history of SUD, active psychiatric disorders, and persistent problematic pain despite appropriate non-opioid therapy.
③ Exclude patients with an active SUD.
④ Stabilize psychiatric disorders before administration of opioids.
⑤ Continue non-opioid medication in CNCP patients with a history of SUD for persistent problematic pain.
⑥ Prescribe an opioid at less than 90 MME/d. When setting an upper limit, 90 MME/d, is better than no limitation.
⑦ Prescribe an opioid at less than 50 MME/d in patients who can understand the risk of an increased dose of opioids.
⑧ Switch to other opioids in patients who have persistent problematic pain and/or adverse reactions.
⑨ Taper opioids to the minimal effective dose in patients who are currently using over 90 MME/d.
⑩ Send patients who have trouble in tapering opioids to a formal multidisciplinary program.
3) Five recommendations from the European Pain Federation position paper in 2017 [30]
4) Fourteen recommendations from the American Pain Society (APS) and the American Academy of Pain Medicine (AAPM) in 2009 [64]
① Consider a trial of opioids when benefits outweigh harms. A benefit-to-harm evaluation is performed by history taking, physical examination, and a risk evaluation for substance abuse, misuse, and addiction. Moderate to severe pain produces decreased function and quality of life.
② Informed consent for opioid therapy includes objectives, expectations, adverse reactions, and other treatment options. A written opioid plan should include responsibilities and expectations for the patient and clinician, as well as patient education.
③ Clinicians and patients should determine whether opioids should be used. Initiation and titration of the opioid should be individualized. There is a lack of evidence for recommending IR versus ER formulations and for as-needed versus around-the-clock dosing.
④ Methadone has confusing and fluctuating pharmacokinetics and pharmacodynamics, requiring careful initiation and titration by physicians who are familiar with its dosage and adverse reactions.
⑤ After opioid medication, intensity of pain, function and quality of life, progression of therapeutic goals, adverse reactions, and adherence, as well as, if necessary, periodic urine drug screening should be monitored and reassessed. Progression notes include the current analgesic regimen, pain and pain relief, daily living activities, adverse reactions, aberrant behaviors, and analgesic plans for opioid therapy.
⑥ More frequent and tighter monitoring, consultation with psychiatry or addiction specialists, and discontinuation of opioids are required in high-risk patients with a history of drug abuse, psychiatric issues, or serious aberrant behaviors.
⑦ Reevaluate the causes of the benefits-to-harms in repeated opioid dose escalations. Consider more frequent follow-up visits when showing adverse reactions with poor health status and adherence to the opioid therapy. Consider opioid rotation for intolerable adverse effects or inadequate benefits despite dose increases. Taper or stop opioid administration in cases of repeated aberrant opioid-related behaviors, opioid diversion/abuse, lack of progress towards therapeutic goals, or intractable adverse reactions.
⑧ Prevent, diagnose, and treat adverse reactions.
⑨ Integrate interdisciplinary or multidisciplinary pain management routinely for CNCP.
⑩ Discuss cognitive impairment owing to opioid therapy with patients.
⑪ Consult and communicate with other clinicians.
⑫ Consider an IR opioid prescription for breakthrough pain during ER opioid therapy for continuous pain after analysis of therapeutic benefit versus risk.
⑬ Persuade pregnant women not to use opioids or to use a minimal dosage of opioids during the intrapartum and postpartum period. Prepare and treat risks to the patient and newborn. OWS can be expected in over 50% of newborns of opioid-dependent mothers.
⑭ Be familiar with laws, regulatory guidelines, and policy statements.
5) The American Society of Addiction Medicine (ASAM) National Practice Guideline for treatment of OUD in 2020
-
① Assessment and diagnosis of OUD: Appropriate referral to an emergency or psychiatric department, general evaluation of the patient for establishment of treatment, history taking, physical examination, laboratory tests with tests for infectious diseases as well as testing for pregnancy, mental health status, and psychiatric disorders, the coexistence of other SUDs, and multidimensional assessment are essential to assess patients with OUD.
Diagnosis of OUD must be obtained from history taking and before pharmacotherapy. It is necessary to perform drug testing for evaluating adherence to prescribed medications as well as for detecting other SUDs. ② Treating OUD: FDA-approved medications and psychological referral can be available based on the PDMP. Methadone is prescribed in opioid treatment programs (OTPs) and acute care settings. Buprenorphine is prescribed by approved clinicians in any setting. However, naltrexone can be prescribed by any clinicians in any setting. Naloxone is also used for reversal of opioid overdose in OUD. MME/d is not applicable to medications for the treatment of OUD.
-
③ Treating OWS: Methadone or buprenorphine is also used for OWS resulting from abrupt cessation of opioids. Detoxification is to manage acute OWS for the prevention of relapse and overdose of opioids. In addition to detoxification, continuing maintenance medication with psychosocial therapy is the standard treatment for OWS.
The initial dose of methadone withdrawing from short-acting opioids is 20–30 mg daily, and tapering off may be completed in 6–10 days. The initial dose of 2–4 mg/d of buprenorphine, followed by titrating up as needed to suppress the OWS, should be used when objective signs of OWS are found. Methadone and buprenorphine, rather than alpha-2 agonists (FDA-approved lofexidine and off-label clonidine), are more effective for OWS. -
④ Methadone: After getting informed consent, the initial dose of methadone starts from 10 to 30 mg. The first dose may be reduced 2.5 to 10 mg for patients with low or no opioid tolerance. Usual daily doses range from 60 to 120 mg after increasing the dose 10 mg every 5 days under monitoring and psychosocial treatment, in order to prevent it leading to misuse or diversion.
Initial dosing and titration are also needed for re-initiation. Prevention of relapse is an essential goal of addiction treatment. Transition from methadone to another medication is a difficult challenge owing to intractable, dangerous adverse reactions, resulting in relapse. Transitioning from less than 30–40 mg per day of methadone to buprenorphine is tolerable. However, transition from methadone to naltrexone requires complete cessation of methadone or other opioids before administration of naltrexone. There is no deadline for methadone therapy. Patients should know the risk of overuse of other opioids or overdose death from illicit opioid use when discontinuing methadone for the treatment of OUD. -
⑤ Buprenorphine: Buprenorphine is a partial mu opioid agonist. It is used for treatment for both OUD (with a similar effect to methadone) and OWS (with a better effect than lofexidine or clonidine). Contraindications include hypersensitivity and severe hepatic impairment. Other SUDs, hypovolemia or use of antihypertensive agents, and severe cardiovascular disorders need attention for the use of buprenorphine. Drug interaction may develop with central nervous system depressants and agents which affect CYP3A4 activity, such as ketoconazole (antifungal agents), erythromycin (macrolide antibiotics), and human immunodeficiency virus protease inhibitors.
FDA warnings on use of all opioids including methadone and buprenorphine include respiratory depression in concomitant use with benzodiazepines, serotonin syndrome in interaction with antidepressants and migraine medicines, Addison’s disease, and decreased sex hormone levels with decreased libido, impotence, or infertility [67].Various FDA-approved buprenorphine formulations are available, including daily sublingual tablets of buprenorphine (monoproduct), daily sublingual tablets or a film combination of buprenorphine and naloxone, monthly or weekly injection of buprenorphine ER, and subcutaneous implants of buprenorphine hydrochloride every 6 months.Initiation starts with a dose of 2–4 mg, increasing the dosage in increments of 2–8 mg. A daily dose of 16 mg or more has been shown to be effective. However, higher doses of more than 24 mg per day do not show greater effectiveness, but instead increase the risk of diversion.Psychosocial treatment is also helpful in the treatment of OUD with buprenorphine. Drug testing is monitored for adherence to buprenorphine and other controlled substances. A transition from buprenorphine to naltrexone needs 7–14 days when there is no longer any physical dependency on opioids.A transition from buprenorphine, a partial agonist, to methadone, a full agonist, does not require a time delay. Buprenorphine can be used any time during the treatment of OUD. Tapering and discontinuation take several months and ongoing monitoring after discontinuation is also essential. -
⑥ Naltrexone: Intramuscular ER naltrexone is more effective for prevention of OUD relapse than oral naltrexone. Oral naltrexone is only effective in some highly motivated and compliant patients under the supervision of their family. Oral naltrexone is administered from 25 mg on the first day, increasing to 50 mg daily from the second day, and followed by a 3-day per week regimen (100-0-100-0-150-0-0 mg, a total of 350 mg weekly).
ER naltrexone is commonly administered by intramuscular injection every 4 weeks with a dose of 380 mg. It is helpful to administer it every 3 weeks in rapid metabolizers. There are 4 goals in naltrexone therapy for OUD: prevention of OUD relapse in detoxified patients who are no longer physically dependent on opioids, blocking illegal opioids, reducing opioid craving, and encouraging patient attendance in recovery programs.Compared to agonist therapy with buprenorphine or methadone, naltrexone can be used in cases of contraindications to buprenorphine or methadone therapy, in those highly motivated to taper off buprenorphine or methadone therapy, in those who have had unsuccessful results with buprenorphine or methadone therapy, and those who refuse buprenorphine or methadone therapy.Before starting naltrexone, administration of IR and ER opioids should be stopped for 6 and 7–10 days, respectively. Because of uncertainty regarding physical dependency on opioids, a short-acting opioid antagonist, naloxone hydrochloride, or a low-dose oral naltrexone, can be initiated.Common adverse reactions of naltrexone include sleeplessness, nervousness, lethargy/sedation, nausea/vomiting, abdominal cramps, chills, headache, arthralgia/myalgia, and injection site pain.Urine drug testing is recommended for the evaluation of adherence for medication and illegal drug use. The frequency of the urine test (at least 8 times a year) is determined by the adherence of the patients with their different medication in their different treatment settings.Transition from naltrexone to buprenorphine or methadone is applied in cases of intolerable adverse reactions, maintenance of unsuccessful treatment goals, and at the demand of the patient. ⑦ Psychosocial treatment: Psychosocial treatment helps patients reduce craving and relapse, so as to cope with the psychosocial challenge. The therapeutic goals of psychosocial treatment are to modify the underlying processes, to encourage participation and adherence to the treatment plan, and to treat any other psychiatric disorders which may make OUD worse or trigger a relapse. Psychosocial treatment includes evaluation of psychosocial requirements, advice, connection to existing support systems, and referral. In patients receiving methadone, buprenorphine, or naltrexone, psychosocial needs are assessed, and referrals are also provided.
-
⑧ Special populations of pregnant women: Obstetrical complications related to OUD include preeclampsia, abortion, premature delivery, and fetal growth retardation and death. Neonatal abstinence syndrome (NAS) is defined as a group of withdrawal signs in infants after exposure to substances (often opioid agonists) prenatally. Neonatal opioid withdrawal syndrome (NOWS) refers to withdrawal signs in infants who have had uterine opioid exposure. The infants may have hyperactive central and autonomic nervous systems, affecting the gastrointestinal and respiratory systems. Symptoms may start from minutes to 2 weeks after birth, but usually within 3 days. The treatment is opioid agonist medication in tapering half doses.
Physical examination in pregnant women includes objective opioid intoxication and OWS. The pregnant women with OUD may seek antenatal care late, show missed appointments, and have insufficient weight gain. Injection drug users may show punctured skin evidence, cellulitis, or abscesses. Laboratory tests includes HIV and viral hepatitis.Pregnant women with active OUD start with methadone or buprenorphine as the choice of treatment, as soon as possible, during pregnancy. It is recommended for them to be hospitalized at the beginning of methadone or buprenorphine treatment to avoid the potential adverse reactions, especially during the third trimester. It is better to start opioid agonist therapy as early as possible because there is little confirmation that methadone or buprenorphine produces higher rates of NOWS. An experienced clinician in both OUD treatment and obstetric care should manage pregnant women with OUD.The initial dose of methadone starts from 10–30 mg, and incremental doses of 5–10 mg every 3–6 hours is recommended for managing OWS. The maximum dose on the first day is 30–40 mg. Every 5 days, the dose can be limited to increase by 10 mg to control OWS with the lowest dose. Plasma levels of methadone progressively decrease but clearance increases, as gestational age advances. Therefore, split doses may be needed as pregnancy progresses. Reduced doses are needed postnatally.Naltrexone should be discontinued after pregnancy. Naloxone is also not recommended. However, breastfeeding mothers are recommended to take methadone or buprenorphine. -
⑨ Special populations of individuals with pain: Alternative treatments, including non-opioid medications (acetaminophen or NSAIDs), behavioral approaches, physical therapy, or regional anesthesia, should be sought first before the use of opioids.
It is advised for patients with pain who have active OUD to use methadone or buprenorphine. Temporarily increasing the dose or dosing frequency is helpful. Patients who treat moderate to severe acute pain with a regular dose of methadone for the treatment of OUD may require a higher dose of a supplemental short-acting full agonist opioid.Rescue doses of buprenorphine in supervised settings, rather than in ambulatory care settings, during hospitalization may have better results in patients receiving buprenorphine for OUD who have moderate to severe acute pain which is refractory to other treatments. It is not necessary to discontinue administration of methadone or buprenorphine preoperatively. It is also allowable to use intravenous strong opioids intraoperatively. Postoperative daily doses can be restarted 3 days after operation.In patients who are taking naltrexone and did not respond to opioid analgesics for their somatic pain, non-opioid analgesics are recommended in cases of mild pain, and higher potency NSAIDs, such as ketorolac, are recommended in moderate to severe pain on a short-term basis. High potency full agonist opioids can overcome a blocking effect of mu opioid receptors from naltrexone. ⑩ Special populations of adolescents: Methadone, buprenorphine, and antagonists can also be used in OUD in adolescents. It is appropriate for patients and their parents to participate the treatment of OUD by both pharmacotherapy and psychosocial treatment. Blood-born infections and sexually transmitted infections should be controlled.
⑪ Special populations with current psychiatric disorders: Suicidal or homicidal ideation in OUD patients with psychiatric disorders should be recognized.
⑫ Special populations in the criminal justice system: It is easy to ignore the forced OWS in individuals entering the criminal justice system. Three FDA-approved medications can be provided to individuals within the criminal justice system and even after release. Naloxone kits are prepared within the criminal justice system.
⑬ Naltrexone for treatment of opioid overdose: Naltrexone for opioid overdose is given in both general patients and pregnant women with OUD. Naloxone can be given by trained family members.
⑭ Areas for further research: Personalized medication with new treatment methods may be advantageous in future studies.
6) OTPs suggested by the United States Substance Abuse and Mental Health Services Administration (SAMHSA) in 2015
5. Screening tests for prevention of OUD
Table 2
| Pre-administration opioid risk evaluation methods | ||||
| 1. Opioid Risk Tool by patients [71] | ||||
| Man | Woman | |||
| Family history of substance abuse | Alcohol | 1 | 3 | |
| Illegal drugs | 2 | 3 | ||
| Prescription drugs | 4 | 4 | ||
| Personal history of substance abuse | Alcohol | 3 | 3 | |
| Illegal drugs | 4 | 4 | ||
| Prescription drugs | 5 | 5 | ||
| Age between 16 and 45 years old | 1 | 1 | ||
| History of pre-adolescent sexual abuse | 3 | 0 | ||
| Psychological diseases | Attention deficit disorder, obsessive compulsive disorder, bipolar disorder, or schizophrenia | 2 | 2 | |
| Depression | 1 | 1 | ||
| Total score (26) | Low risk (0–3) | |||
| Moderate risk (4–7) | ||||
| High risk (≥ 8) | ||||
| 2. The Screener and Opioid Assessment for Patients with Pain-Revised (SOAPP-R) by patients [72] | ||||
| 3. The Screening Instrument for Substance Abuse Potential (SISAP) by patients [73] | ||||
| 4. The Diagnosis, Intractability, Risk, and Efficacy (DIRE) score by clinicians [74] | ||||
| (1) Diagnosis | 1 = Benign chronic condition with minimal objective findings or no definite medical diagnosis | Fibromyalgia, migraine, or non-specific back pain | ||
| 2 = Slow progressive condition concordant with moderate pain, or fixed condition with moderate objective findings | Failed back surgery syndrome, back pain with moderate degenerative changes, neuropathic pain | |||
| 3 = Advanced condition concordant with severe pain with objective findings | Advanced neuropathy, severe spinal stenosis | |||
| (2) Intractability | 1 = Trial of few therapies and a passive role in patient’s pain management process | |||
| 2 = Trial of most customary treatments, but partially engaged in patient’s pain management process | ||||
| 3 = Trial of appropriate treatment, but inadequate response | ||||
| (3) Risk | Psychological | 1 = Serious personality dysfunction or mental illness interfering with care | Personality disorder, severe affective disorder, or significant personality issues | |
| 2 = Moderate personality or mental health | Depression or anxiety disorder | |||
| 3 = Good communication with clinic | No significant personality dysfunction or mental illness | |||
| Chemical health | 1 = Active or very recent use of illicit drugs, excessive alcohol, or prescription drug abuse | |||
| 2 = Chemical coper or history of chemical dependency in remission | ||||
| 3 = No chemical dependency history | ||||
| Reliability | 1 = History of numerous problems: medication misuse, missed appointments, rarely follows through | |||
| 2 = Occasional difficulties with compliance, but generally reliable | ||||
| 3 = Highly reliable patient with medications, appointments, and treatment | ||||
| Social support | 1 = Life in chaos, little family support and few close relationships, loss of most normal life roles | |||
| 2 = Reduction in some relationships and life roles | ||||
| 3 = Supportive family/close relationships. Involved in work or school and no social isolation. | ||||
| (4) Efficacy | 1 = Poor function or minimal pain relief despite moderate to high doses. | |||
| 2 = Moderate benefit with function improved in a number of ways or insufficient information | ||||
| 3 = Good improvement in pain and function and quality of life with stable doses over time | ||||
| Total DIRE score (21) | Score 7–13 | Not a suitable candidate for long-term opioid analgesia | ||
| Score 14–21 | A suitable candidate for long-term opioid analgesia | |||
| Intra-administration opioid risk evaluation methods | ||||
| 1. Prescription Drug Use Questionnaire-patient version (PDUQ-p) by patients [75] | ||||
| 2. Current Opioid Misuse Measure (COMM) by patients in the past 30 days [76] | ||||
| 6 Concept Map Clusters | 17 Items | Never (0), Seldom (1), Sometimes (2), Often (3), or Very Often (4) | ||
| (1) Signs and symptoms of drug misuse | Trouble with thinking clearly or memory problems | |||
| (2) Emotional problems/psychiatric issues | Complaints from others about incompletion of necessary tasks | |||
| Serious thought about self-harm | ||||
| Arguing with others | ||||
| Trouble managing your anger | ||||
| Experiencing anger with people | ||||
| (3) Poor response to medications | ||||
| (4) Evidence of lying and illicit drug use | Taking medications differently from being prescribed, | |||
| Time spent thinking about opioid medications | ||||
| Taking others’ pain medication | ||||
| Concern about managing your medications | ||||
| Others’ worry about your handling your medications | ||||
| (5) Inconsistent appointment patterns | Visiting multiple providers to get sufficient pain relief | |||
| Making an emergency call or showing up at the clinic without an appointment | ||||
| Visiting an visiting emergency room | ||||
| (6) Medication misuse/abuse as well as noncompliance with medication | Needing to take more of your medication than prescribed | |||
| Borrowing pain medication from others | ||||
| Using pain medication for non-prescribed symptoms | ||||
| Total score | /68 | |||
| A score of 9 or greater out of a total score of 68 is suggestive of current problematic drug-related behaviors. | ||||
| 3. Patient Medication Questionnaire (PMQ) by patients [77] | ||||
| 4. Prescription Drug Use Questionnaire-clinician version (PDUQ-c) by clinicians [78] | ||||
| 5. Pain Assessment and Documentation Tool (PADT) by clinicians [79] | ||||
| 6. Addiction Behavior Checklist (ABC) by clinicians [80] | ||||




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download