Abstract
While the route, location, and pathology of the lingual nerve has been detailed extensively in reports in the literature, its terminal branch to the sublingual gland is often overlooked. It is known, via both gross and histological observation, that the sublingual glandular branch terminates at the posterior aspect of the sublingual gland. Upon routine cadaveric dissection of a male cadaver, one of the lingual nerve branches was found to terminate at the anteroinferior portion of a herniated sublingual gland. This specific course has not previously been discussed or reported via gross or histological observation. Therefore, a timely review of the lingual nerve’s terminal sublingual glandular branch’s anatomy and clinical significance pertaining to this case is warranted. Surgeons who treat patients with submental masses should be aware of the anatomy of this nerve and the potential variance described here in order to avoid postprocedural complications.
The lingual nerve (LN) is a posterior branch of the mandibular branch of the trigeminal nerve [1]. The terminal sublingual glandular branch of the LN carries within it both taste fibers and parasympathetic innervation to the sublingual gland (SLG) via the chorda tympani. Originating from the superior salivary nucleus, the preganglionic parasympathetic fibers exit the skull base and join the LN via the chorda tympani branch of the facial nerve in the infratemporal fossa. In this region, the LN courses anteromedial to the inferior alveolar nerve and runs below the superior pharyngeal constrictor muscle to reach the floor of the mouth [2-4]. The preganglionic fibers of the chorda tympani then synapse in the submandibular ganglion, where they typically distribute postganglionic fibers to the posterior aspect of the SLG. Interestingly in this case report, the LN terminated at the anteroinferior SLG which herniated through the mylohyoid muscle (MHM). This is a previously unreported anatomical variation. The sublingual glandular branch’s position deep to the tongue is well known to physicians as a common location of iatrogenic injury [5]; however, there are only limited available resources regarding anatomical variations. Here, we present a case of the variant large sublingual glandular branch of the LN.
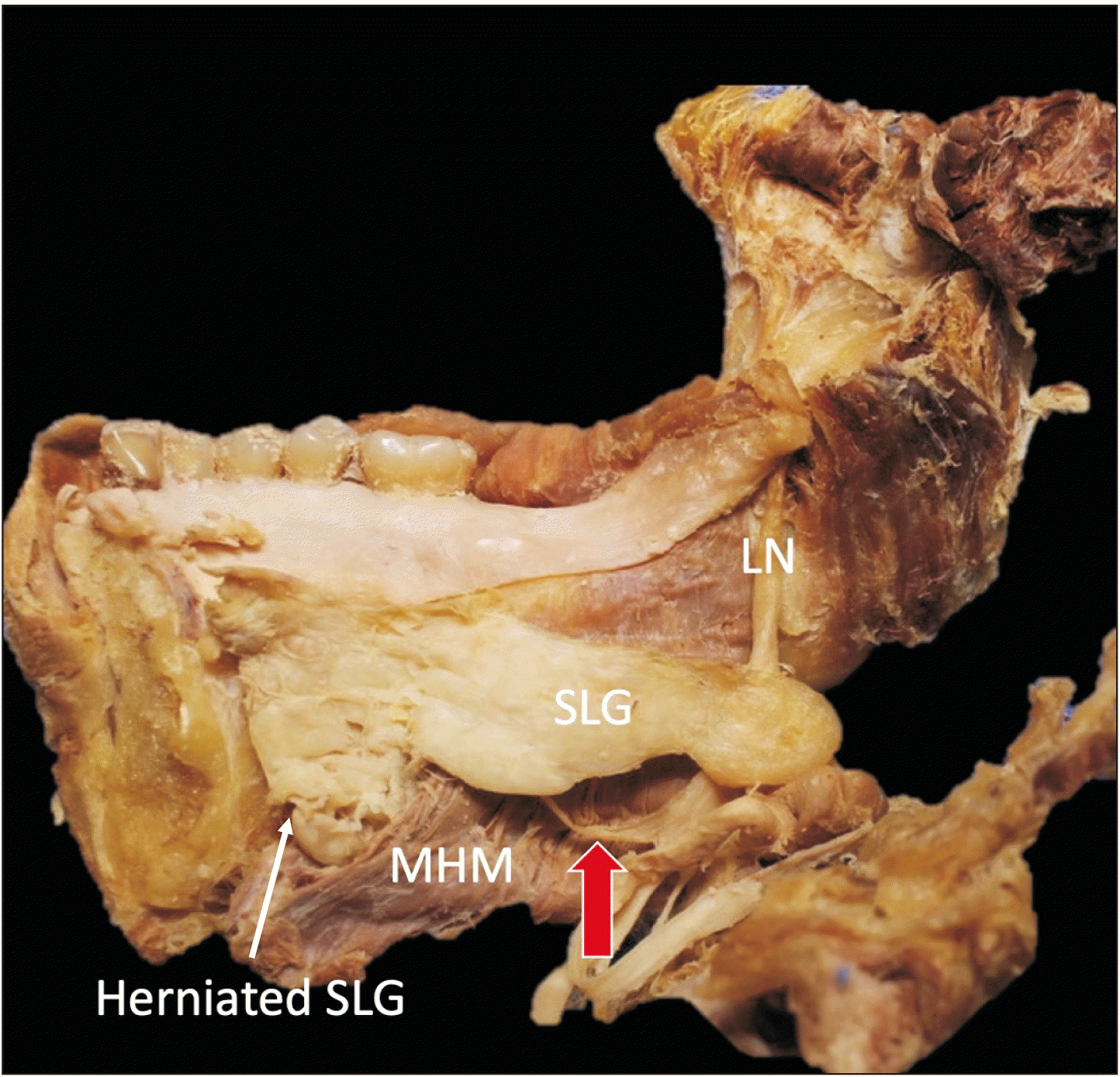
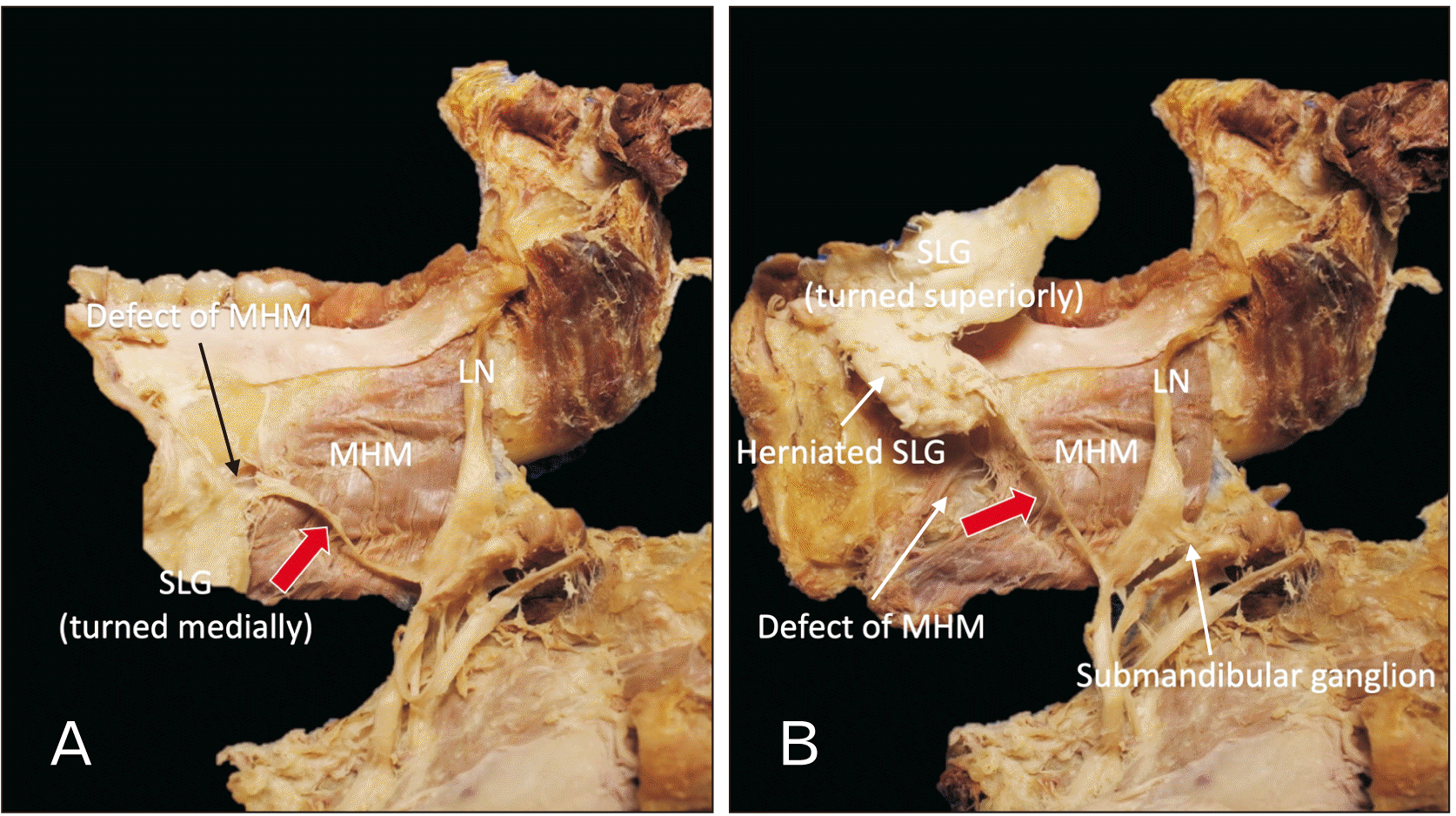
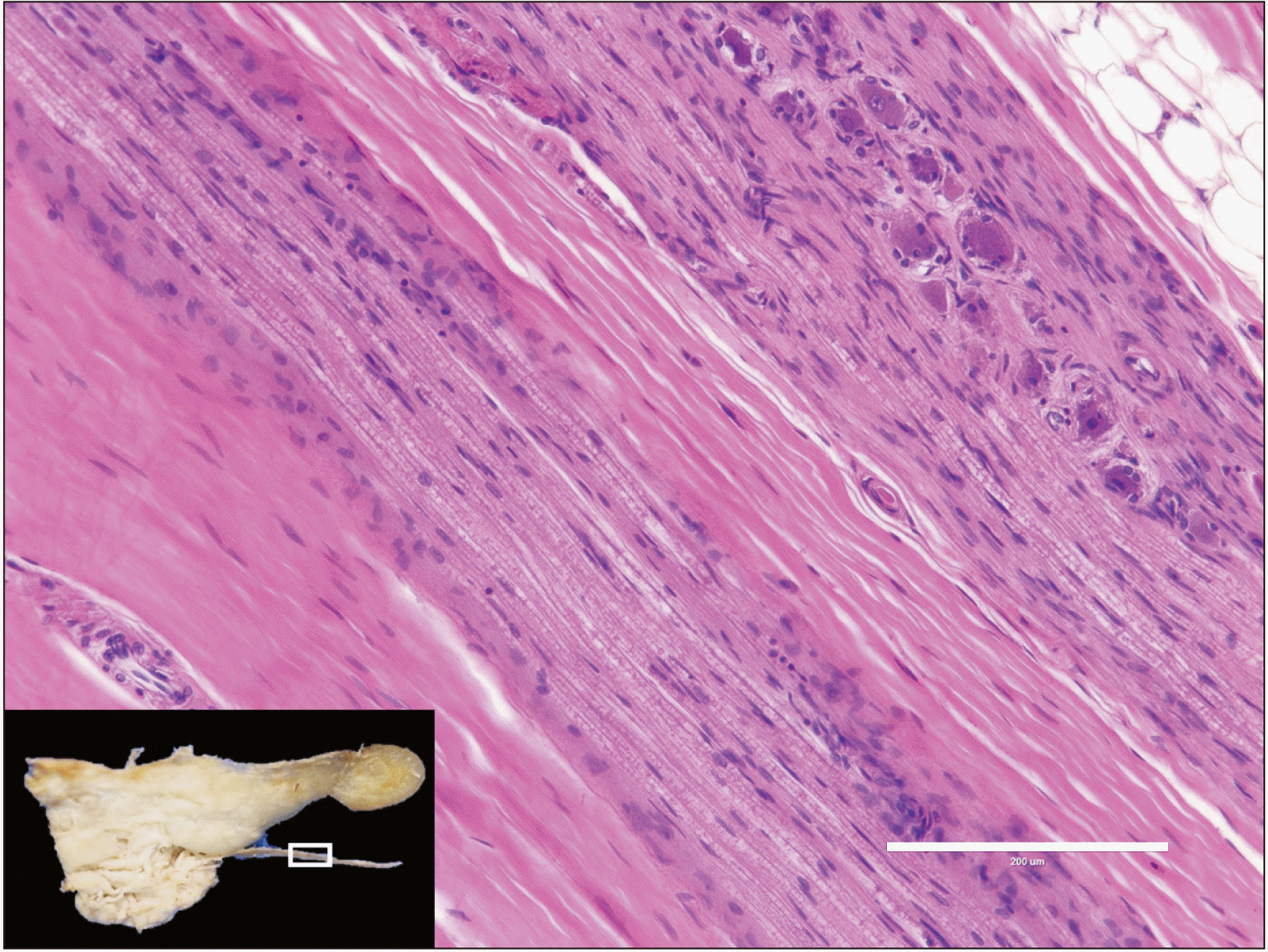
During a routine dissection of an embalmed Caucasian male cadaver whose age at death was 92 years, we observed a large branch arising from the LN which innervated a herniated portion of the SLG (Fig. 1). Specifically, the inferior portion of the right SLG herniated through the MHM to the submandibular space. As exiting the pterygomandibular space, the LN was observed to travel on the surface of the MHM and below the posterior part of the SLG (more specifically, below a small groove on the inferior border of the SLG). Before reaching the SLG, slightly lateral to the SLG, the LN gave off a relatively thick (approximately 2 mm in diameter) branch that terminated at the anteroinferior part of the SLG (herniated part). Termination of the branch was into the submandibular space (Fig. 2). The fibrous tissue was excised in order to observe it histologically (Fig. 3). Histological observation with hematoxylin and eosin staining revealed that the tissue was nerve tissue. No SLG hernia or SLG innervated by the large LN branch was observed on the left side of this specimen. No signs of postoperative scar were observed. This study followed the guidelines of the Declaration of Helsinki.
The LN is well known to physicians due to its variable positioning in the oral cavity, which lends itself to a range of iatrogenic injuries. Anatomically, both the submandibular gland (SMG) and SLG are innervated by the postganglionic parasympathetic fibers of the chorda tympani. There are reports showing variant innervation of the SMG, e.g., dual innervation of the SMG by chorda tympani and the nerve to the MHM [6]. However, we were not able to find reports of variant innervation of the SLG in the existing literature. Unique to this report, is the analysis of a variable pathway of the LN’s terminal branch, the sublingual glandular branch. The LN has been well documented as receiving parasympathetic postganglionic fibers from the chorda tympani approximately 1cm below the bifurcation of the lingual and inferior alveolar nerves [7]. As the LN courses anteroinferior to the lateral aspect of the tongue, it continues deep (superior) to the SMG. Here, the lingual fibers leave the deep (superior) aspect of the SMG and synapse on the submandibular ganglion. Postganglionic parasympathetic in nature, the fibers arising from this ganglion innervate both the SLG and SMG. Upon their termination, these fibers are best known as the sublingual glandular branch of the LN [7]. To our knowledge, this case is the first report of a branch to the SLG traversing a defect in the MHM to supply a herniated SLG.
Whether SLG herniation via a MHM defect is the critical factor in the formation of a hypertrophied branch to the SLG is not clear. The SLG are located superiorly to the MHM. Although close in proximity, the developing SLG differs embryologically from the MHM. The SLG arises from the first and second pharyngeal arches as epithelial buds which, through epithelial to mesenchymal signaling, partake in the branching morphogenesis by weeks eight to nine of development [8]. The mesenchyme of the MHM arises from the mesoderm of only the first pharyngeal arch; however, the muscle is composed of two halves, anterior and posterior, which typically overlap. When properly overlapping, the muscle creates the floor of the oral cavity in addition to separating the superomedial sublingual and inferolateral submandibular spaces [9]. With dehiscence of this muscle, the SLG may traverse this defect.
SLG herniations are very common but have been reported with varying incidences in reports in the literature. This is perhaps due to the asymptomatic nature of this defect. In CT studies, White et al. [10] identified asymptomatic dehiscence of the MHM in 77 out of 100 of scanned individuals, with 37 containing accessory salivary tissue. While asymptomatic, a quarter of such herniations have been reported as palpable upon physical examination [11] and are disproportionally observed in females [12]. Due to their reportedly common prevalence, knowledge of accessory SLG anatomy and herniation with MHM dehiscence should therefore be considered during the examination of a submental mass.
Interestingly, herniated SLGs have been shown to be highly susceptible to subclinical trauma. Specifically, the trio of herniated SLGs, MHM defects, and submandibular fluid collection can result in the blockage of gland tubules resulting in a characteristic swelling called a plunging ranula [13]. Once confirmed via imaging, sialoadenectomy is the recommended treatment modality [14]. This procedure is a transoral excision of the SLG with drainage of cystic contents. Relevant to this report, is the variable termination of the sublingual glandular branch of the LN. Therefore, surgeons should be aware of injuring the sublingual glandular branch of the LN during intraoral salivary gland surgeries, specifically sialoadenectomies, especially when a variant as reported here is present.
Lastly, not all submental neck masses are the result of herniated SLGs. The sublingual glandular branch of the LN can be the site of rare intraoral schwannomas which can form long-standing, firm masses in the submental space. Schwannomas are benign neurogenic neoplasms, which form most typically in the flexor surfaces of the body. Tumors of this nature in the submandibular region are very rare, with only a few being noted in the LN [15], or surrounding anatomy.
Acknowledgements
The authors sincerely thank those who donated their bodies to science so that anatomical research could be performed. Results from such research can potentially increase mankind’s overall knowledge that can then improve patient care. Therefore, these donors and their families deserve our highest gratitude.
Notes
References
1. Standring S. 2020. Gray's anatomy. 42nd ed. Elsevier;London:
2. Kikuta S, Iwanaga J, Kusukawa J, Tubbs RS. 2019; An anatomical study of the lingual nerve in the lower third molar area. Anat Cell Biol. 52:140–2. DOI: 10.5115/acb.2019.52.2.140. PMID: 31338230. PMCID: PMC6624336.

3. Iwanaga J, Cleveland MK, Wada J, Tubbs RS. 2020; How to avoid iatrogenic lingual nerve injury in the retromolar area: an anatomical study of retromolar pad and lingual nerve. Surg Radiol Anat. 42:523–8. DOI: 10.1007/s00276-020-02422-w. PMID: 31989215.

4. Iwanaga J, Nakamura K, Alonso F, Kirkpatrick C, Oskouian RJ, Watanabe K, Tubbs RS. 2018; Anatomical study of the so-called "retromolar gland": distinguishing normal anatomy from oral cavity pathology. Clin Anat. 31:462–5. DOI: 10.1002/ca.23047. PMID: 29349817.

5. Graff-Radford SB, Evans RW. 2003; Lingual nerve injury. Headache. 43:975–83. DOI: 10.1046/j.1526-4610.2003.03189.x. PMID: 14511274.

6. Ryumon S, Hage D, Ibaragi S, Okui T, Tubbs RS, Iwanaga J. 2021; Dual innervation of the submandibular gland by nerve to mylohyoid and chorda tympani. Morphologie. 105:316–8. DOI: 10.1016/j.morpho.2020.11.004. PMID: 33288421.

7. Sittitavornwong S, Babston M, Denson D, Zehren S, Friend J. 2017; Clinical anatomy of the lingual nerve: a review. J Oral Maxillofac Surg. 75:926.e1–926.e9. DOI: 10.1016/j.joms.2017.01.009. PMID: 28189657.

8. Holmberg KV, Hoffman MP. 2014; Anatomy, biogenesis and regeneration of salivary glands. Monogr Oral Sci. 24:1–13. DOI: 10.1159/000358776. PMID: 24862590. PMCID: PMC4048853.

9. Otonari-Yamamoto M, Nakajima K, Tsuji Y, Otonari T, Curtin HD, Okano T, Sano T. 2010; Imaging of the mylohyoid muscle: separation of submandibular and sublingual spaces. AJR Am J Roentgenol. 194:W431–8. DOI: 10.2214/AJR.09.3516. PMID: 20410390.

10. White DK, Davidson HC, Harnsberger HR, Haller J, Kamya A. 2001; Accessory salivary tissue in the mylohyoid boutonnière: a clinical and radiologic pseudolesion of the oral cavity. AJNR Am J Neuroradiol. 22:406–12. PMID: 11156791. PMCID: PMC7973941.
11. Engel JD, Harn SD, Cohen DM. 1987; Mylohyoid herniation: gross and histologic evaluation with clinical correlation. Oral Surg Oral Med Oral Pathol. 63:55–9. DOI: 10.1016/0030-4220(87)90340-9. PMID: 3468466.

12. Yang HC, Kim SY, Kim SK, Oh CS, Chung IH, Nam KI. 2016; A cadaveric study on mylohyoid herniation of the sublingual gland. Eur Arch Otorhinolaryngol. 273:4413–6. DOI: 10.1007/s00405-016-4095-1. PMID: 27180250.

13. Jain P, Jain R. 2014; Types of sublingual gland herniation observed during sonography of plunging ranulas. J Ultrasound Med. 33:1491–7. DOI: 10.7863/ultra.33.8.1491. PMID: 25063415.

14. Shakeel M, Nair S, Ahmad Z. 2016; Painless, reproducible, reversible floor of mouth herniation: what's in there? J Otolaryngol ENT Res. 4:00118. DOI: 10.15406/joentr.2016.04.00118.

15. Attie JN, Friedman E, Rothberg MS. 1984; Submandibular and axillary schwannomas not associated with von Recklinghausen's disease. J Oral Maxillofac Surg. 42:391–4. DOI: 10.1016/S0278-2391(84)80012-9. PMID: 6425471.

Fig. 1
A large branch (red arrow) of the LN running lateral to the SLG. LN, lingual nerve; MHM, mylohyoid muscle; SLG, sublingual gland.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download