Abstract
The yolk sac is supplied by the vitelline artery and vein (VA, VV), which run through the yolk stalk in combination with the omphaloenteric duct. Moreover, the VV takes a free posterior course outside the midgut mesentery containing the secondarily-developed superior mesenteric vein (SMV). However, the regression process of these structures has not been demonstrated photographically. The present study evaluated serial histological sections from 20 embryos of stages 15–19 or crown-rump length (CRL) 7.5–20 mm. All specimens carried the SMV as sequential tissue slits. However, an omphaloenteric duct with epithelia continuous with the midgut loop was not observed. In smaller embryos (CRL <13 mm) the VA extended distally or anteriorly from the midgut apex in the extra-embryonic coelom, whereas in larger embryos (CRL 16–20 mm) the artery was absent from the distal side of the apex. The entire course or part of the VV outside the mesentery was always seen, but four larger embryos lacked the venous terminal near the duodenum. A vacuole-like remnant of the yolk sac was present in all smaller embryos (CRL <10 mm), but was absent from 7 of the 11 larger embryos. The size of the remnant was equal to the thickness of the VA or VV, with the remnant being sandwiched between the VA and VV. Moreover, the regressing yolk sac often communicated with or opened to the VV. Consequently, the yolk sac regressed first, followed by the regression of the VA until 6 weeks. The yolk stalk was clearly observed until 5 weeks.
In contrast to the superior mesenteric vein (SMV), which develops secondarily in the midgut mesentery without contribution from the vitelline vein (VV), the vitelline or omphalomesenteric artery is thought to directly transform into the superior mesenteric artery (SMA) [1, 2]. Although the development and growth of the midgut loop and its blood supply and drainage have been major interests in embryology [3-5], less is known about the upstream or extra-embryonic portion of the vitelline artery (VA) and VV. Similarly, there have been few photographic evaluations of yolk sac morphology in human embryos. A digitized series of two digitally reproduced embryonic morphology (DREM) embryos of Carnegie stages (CS) 13–18 (http://virtualhumanembryo.lsuhsc.edu/) show both the VA and VV running freely outside the mesentery in the extra-embryonic coelom or herniation sac. However, the posterior termini of the candidate VA and VV were not clearly visible in these virtual embryos.
Micro-computed tomography has shown that, following “regression of the omphaloenteric duct” in rat embryos at ED12, a combination of the VA and VV maintains its long course through the yolk stalk in the extra-embryonic coelom until ED13 or later [6]. In addition, that study showed a switching of midgut venous drainage in the extra-embryonic coelom from short tributaries of the VV (ED12) to an adult-like long SMV (ED13). Notably, the contents of the yolk stalk seemed to differ between mouse and human embryos at the same stage. An excellent series of 3D reconstructions of human embryos has shown that the yolk stalk contains the omphaloenteric duct as well as the vitelline vessels at CS 14–18 (approximately 4–6 weeks) [2]. Therefore, at CS 19–20 or approximately 6 weeks, the human VA seemed to transform into the SMA without a distal course outside the midgut mesentery.
The human yolk sac seems to be much smaller than that of experimental animals at the same stage [7, 8]. To our knowledge, to date there has been no histological demonstration of the human yolk sac supplied with the VA and VV. Because of the extreme difficulty of finding histological sections of human embryos at 3–4 weeks, we assessed 20 specimens of CS 15–19 (approximately 5–6 weeks) to provide photographic evidence of 1) candidates for the yolk sac remnant, 2) a regressing VA and a persistent VV in the extra- and intra-embryonic coelom, and 3) the topographical anatomy of the most anterior portion or apex of the midgut loop (midgut apex), including the omphaloenteric duct.
This study was performed in accordance with the Declaration of Helsinki 1995 (as revised in 2013). Paraffin-embedded histological sections were obtained from 20 embryos of stages 15–19 [9] and crown-rump length (CRL) 7.5–20 mm (approximately 5–6 weeks). The sectional planes of 14 specimens were horizontal, whereas the planes of the other six specimens were sagittal. All sections were part of the large collection maintained by the Department of Anatomy of the Universidad Complutense, Madrid, Spain, with these embryos being the products of miscarriages and ectopic pregnancies obtained from the Department of Obstetrics of the University. No information was available on the genetic background of the embryos and/or the cause of abortion. The sections were stained with hematoxylin and eosin (H&E) or Azan. This study was approved by the Ethics Committee of Complutense University (B08/374). All photographs were taken with a Nikon Eclipse 80 (Nikon, Tokyo, Japan). The identification of the intestines on each section was based on our previous studies [10-12].
Figs. 1–7 are arranged in order from smaller to larger specimens, although this order did not always correspond to the stage; for example, the embryo in Fig. 2D–E is younger than that in Fig. 2A–C according to the morphologies of the heart and liver. Since all seven specimens shown in Figs. 1–6 were at 5 weeks of gestation, a figure-to-figure explanation is necessary at the head of this section for introduction of the individual difference in morphology. Regardless of whether the umbilical cord containing the extra-embryonic coelom protruded superiorly or inferiorly, the upstream portion near the placenta was described as the “distal” portion. Thus, the VA and VV, along with the umbilical vessels, protruded distally beyond the midgut apex. When the dorsal, intramesenteric course of the VA was called “SMA”, the VA was regarded as a free distal artery outside the mesentery. The sagittal sections of six specimens (Table 1) were unlikely to contain a distal part of the VA, possibly due to tissue injury during the histological procedure.
Fig. 1 may be most difficult to understand the topographical anatomy because abdominal structures are packed in an extremely narrow space. However, as a diagram summarizes (Fig. 1K), a small vacuole-like yolk sac is surrounded by a VV and two branches of the VA. Fig. 2, sagittal sections containing two embryos, exhibits a fact that the yolk sac remnant is likely to exist near the heart and liver or in the dorsal site in the intra-embryonic celom. Fig. 3 may be easy to read because of the large celomic cavity. The yolk sac remnant is seen in the extra-embryonic celom (Fig. 3A–C) and the VV runs freely in the celom to approach the duodenum (Fig. 3E, F). Although Fig. 4 is composed of many panels, a half of them (Fig. 4A–F) shows details of a close relation, even a communication, between the VV and yolk sac (summarized in Fig. 4L). Although the yolk sac remnant is absent, Fig. 5 is characterized by 1) the dilated veins (Fig. 5B–E) and 2) a rarely long, longitudinal cut of the VA (Fig. 5G). Fig. 6 focuses the VV and VA in the extra-embryonic celom: they have free ends in the celom without any yolk stalk-like structure (Fig. 6F). Finally, Fig. 7 shows a limited 6 weeks’ embryo that had a yolk sac remnant in spite of the large body size (summarized in Fig. 7A).
In all specimens, the midgut apex reached the extra-embryonic coelom in or adjacent to the umbilical cord. Candidate yolk sac remnants were found in 13 of the 20 specimens, although these remnants in horizontal sections were usually small, round vacuole-like structures. The yolk sac remnant often communicated with (Fig. 4E) or opened to (Fig. 7F) the VV or SMV. In smaller specimens (CRL <12 mm), the yolk sac remnant was present on the distal side of the midgut apex, but, in larger specimens (CRL >16 mm), the remnant was located on the proximal or posterior side of the apex. In sections containing a yolk sac remnant, the yolk stalk was thick and easy to identify (Figs. 3A, 4A). However, the yolk stalk on sagittal sections was often unclear, possibly due to oblique cutting (Fig. 2A–C). The SMV was always present, usually as a series of irregularly-shaped slits of the mesentery tissue (Figs. 1J, 3G, and 5B). Thus, the luminal surface of the SMV was not smooth. We did not found candidates of the epithelial-lined omphaloenteric duct originating from the midgut apex and extending distally along or near the VA and/or VV.
The VA in the extra-embryonic coelom of the umbilical cord usually ran distally along the yolk stalk on the distal side of the midgut apex, with or without the concomitant VV (Figs. 1C and 3B, 4C, 5A–D). In larger specimens, however, the VA was absent, being replaced by the SMA within the mesentery (Fig. 7D, G, Table 1). In the larger specimens, strictly, the VA regressed in the distal side of the midgut apex and the proximal long portion remained as a SMA. The VV was present in all specimens, irrespective of whether its course was partial or complete, with the posterior ending of the VV at the mesentery near the duodenum being present in all of the smaller specimens (Figs. 3F, 4J and 5D and 7G). Therefore, the VV was likely to regress in the posterior or proximal course. Conversely, in the extra-embryonic coelom, the VA started to regress earlier than the VV. Branches of the VA and tributaries of the VV were rarely observed near the yolk sac remnant in the yolk stalk (Fig. 1A). Sagittal sections showed that the VV curved around the duodenum, resulting in a connection between the pre- and post-duodenal portal veins (Fig. 2B, C).
Overall, a vacuole-like remnant of the yolk sac was consistently present in embryos smaller than 10 mm CRL, but was absent from 7 of the 11 larger embryos (11–20 mm CRL). The VA disappeared in the distal side of the midgut apex in larger embryos (>16 mm CRL), but the VV was likely to still remain. Fig. 8A is a diagram showing a whole embryo at 5 weeks with a set of the vitelline vessels and yolk sac remnant: the sac was thinner than the umbilical artery and vein and it had lost a capillary network for the vascular supply. At and until 6 weeks, The VA and yolk sac were almost always lost, while the VV still ran freely through the extra-embryonic celom or herniation sac (Fig. 8B). Therefore, Fig. 7 exhibits a rare specimen containing the yolk sac remnant even at 6 weeks.
Although the number of specimens examined was limited, the present study was likely the first systematic examination of the upstream portions of the VA and VV in human embryos. The yolk sac remnant was strikingly small and sandwiched between the VA and VV at the distal end of the yolk stalk. This was quite different from the classical image of a bulky yolk sac surrounding blood capillaries at 5–6 weeks in textbooks of embryology. In their excellent 3D-diagrams, Hikspoors et al. [4] showed multiple tributaries of the VV surrounding and draining the yolk sac at CS 11 and 12. According to them, however, any of such tributaries seemed to disappear suddenly at CS 13 or within approximately 5 weeks. To our regret, we have no available histological information before and at the drastic change. In addition, we ensured a fact that the superior mesenteric vein develops secondarily as a sequence of tissue slits in the mesentery.
The present study also demonstrated a considerable difference in topographical anatomy of the VA, VV and yolk sac remnant in embryos at 5 weeks. In short, this seemed to be a result of extremely rapid changes of the stage. In spite of the almost same size, some embryos were characterized by long courses of the VV and VA, while the others had the vessels and midgut loop packed tightly in a narrow celomic cavity. Likewise, whether the vessels were thin or dilated also provided different morphologies. Thus, in addition to a great contribution of the increased length of intestines, the vascular dilation might increase a volume of the extra-embryonic celomic cavity. A key factor for understanding the different anatomy seemed to be the yolk sac: the regression occurs first in the yolk sac, and subsequently the VA seemed to disappear from the distal end. Growth of the midgut loop might facilitate the VA regression.
The secondary yolk sac originates from membrane folds of the primary, large sac [13, 14] and is followed by remodeling of the vitelline vessels [15]. In human embryos, this remodeling may drastically reduce vascular distribution around the secondary yolk sac. Being different from the initial sac opening to the midgut lumen [4, 16], the secondary yolk sac membrane is not continuous with the midgut epithelium [17]. This may explain the absence of the omphaloenteric duct in the present specimens. The so-called omphaloenteric duct in humans was most likely to disappear at 4 weeks. However, our observations could not rule out the possibility of “another” connection after 6 weeks between the midgut apex and umbilical cord. We suspected the usual explanation of a famous Meckel’s diverticulum of intestine that should be derived from the initial omphaloenteric duct.
The VA was found to disappear earlier than the VV on the distal side of the midgut apex. We have a series of unpublished data showing a free VV outside of the mesentery even at 9–10 weeks. Actually, a persisting VV has been shown to form a strangulating loop around the intestine [18]. Although tributaries of the VV were not evident in the present specimens, left and right VVs are likely to co-exist. Similarly, because branches of the VA supply the initial yolk sac, multiple VAs are likely to be present even after regression of the sac. These branches, however, seemed not to correspond to multisegmental vitelline VAs of the type often shown in reviews [19] and textbooks [1]. Photographic evaluation of mouse embryos showed the presence of multisegmental arterial roots from the aorta at E 9.5–10.5 [20].
Many recent studies have shown persistent vitelline vessels running along the umbilical cord surface at birth. However, it is difficult to determine whether these thin vessels are actual remnants or develop secondarily as collaterals of the embryonic umbilical vein [19, 21, 22]. These veins in adults, have sometimes been called umbilical veins [23], resulting in serious confusion between embryonic umbilical and vitelline veins. Most of these adult veins seem to correspond to paraumbilical veins [24, 25].
Finally, we considered small numbers of sagittal sections at 5 weeks as the greatest limitation of this study. Although the numbers were limited, the present sagittal sections suggested, within 5 weeks, the yolk sac was likely to exist in the intra-embryonic cavity or even in the dorsal site below the liver and heart, not in the extra-embryonic celom in the ventral side of the future umbilicus. However, the present sagittal sections did not demonstrate the ventral abdomen because of the tissue injury.
Acknowledgements
This study was supported by Wuxi Municipal Bureau on Science and Technology (N20202008) and Zhangjiagang Science and Technology Innovation Project (ZKCXY2123) in China.
Notes
References
1. Keibel F, Mall FP. 1912. Manual of human embryology. Vol. 2:Lippincott;Philadelphia: p. 587–659.
2. Soffers JH, Hikspoors JP, Mekonen HK, Koehler SE, Lamers WH. 2015; The growth pattern of the human intestine and its mesentery. BMC Dev Biol. 15:31. DOI: 10.1186/s12861-015-0081-x. PMID: 26297675. PMCID: PMC4546136.

3. Ingalls NW. 1908; A contribution to the embryology of the liver and vascular system in man. Anat Rec. 2:338–44. DOI: 10.1002/ar.1090020804.

4. Hikspoors JPJM, Peeters MMJP, Mekonen HK, Kruepunga N, Mommen GMC, Cornillie P, Köhler SE, Lamers WH. 2017; The fate of the vitelline and umbilical veins during the development of the human liver. J Anat. 231:718–35. DOI: 10.1111/joa.12671. PMID: 28786203. PMCID: PMC5643923.

5. Nagata A, Hatta S, Ji X, Ishikawa A, Sakamoto R, Yamada S, Imai H, Matsuda T, Takakuwa T. 2019; Return of the intestinal loop to the abdominal coelom after physiological umbilical herniation in the early fetal period. J Anat. 234:456–64. DOI: 10.1111/joa.12940. PMID: 30681143. PMCID: PMC6422813.

6. Ginzel M, Martynov I, Haak R, Lacher M, Kluth D. 2021; Midgut development in rat embryos using microcomputed tomography. Commun Biol. 4:190. DOI: 10.1038/s42003-021-01702-4. PMID: 33580156. PMCID: PMC7881192. PMID: 4c21df4743394e35844de423df32e101.

7. Downs KM, Rodriguez AM. 2020; The mouse fetal-placental arterial connection: a paradigm involving the primitive streak and visceral endoderm with implications for human development. Wiley Interdiscip Rev Dev Biol. 9:e362. DOI: 10.1002/wdev.362. PMID: 31622045.

8. Ross C, Boroviak TE. 2020; Origin and function of the yolk sac in primate embryogenesis. Nat Commun. 11:3760. DOI: 10.1038/s41467-020-17575-w. PMID: 32724077. PMCID: PMC7387521. PMID: 1598664c641746069a6eb00a34244aed.

9. O'Rahilly R, Müller F. 1987. Developmental stages in human embryos. Carnegie Institution;Washington, DC:
10. Cho BH, Kim JH, Jin ZW, Wilting J, Rodríguez-Vázquez JF, Murakami G. 2018; Topographical anatomy of the intestines during in utero physiological herniation. Clin Anat. 31:583–92. DOI: 10.1002/ca.22996. PMID: 29044646.

11. Kim JH, Jin ZW, Murakami G, Chai OH, Rodríguez-Vázquez JF. 2018; Persistent right umbilical vein: a study using serial sections of human embryos and fetuses. Anat Cell Biol. 51:218–22. DOI: 10.5115/acb.2018.51.3.218. PMID: 30310717. PMCID: PMC6172587.

12. Kim JH, Jin ZW, Shibata S, Murakami G, Hayashi S, Rodríguez-Vázquez JF. 2020; Vermiform appendix during the repackaging process from umbilical herniation to fixation onto the right posterior abdomen: a study of human fetal horizontal sections. Clin Anat. 33:667–77. DOI: 10.1002/ca.23484. PMID: 31576606.

13. Luckett WP. 1978; Origin and differentiation of the yolk sac and extraembryonic mesoderm in presomite human and rhesus monkey embryos. Am J Anat. 152:59–97. DOI: 10.1002/aja.1001520106. PMID: 98035.

14. Jollie WP. 1990; Development, morphology, and function of the yolk-sac placenta of laboratory rodents. Teratology. 41:361–81. DOI: 10.1002/tera.1420410403. PMID: 2187257.

15. Garcia MD, Larina IV. 2014; Vascular development and hemodynamic force in the mouse yolk sac. Front Physiol. 5:308. DOI: 10.3389/fphys.2014.00308. PMID: 25191274. PMCID: PMC4138559.

16. Hamilton WJ, Boyd JD, Mossman HW. 1978. Human embryology. 4th ed. Macmillan;London: p. 105p. 272–7. p. 331DOI: 10.3389/fphys.2014.00308.
17. Mançanares CA, Leiser R, Favaron PO, Carvalho AF, Oliveira VC, Santos JM, Ambrósio CE, Miglino MA. 2013; A morphological analysis of the transition between the embryonic primitive intestine and yolk sac in bovine embryos and fetuses. Microsc Res Tech. 76:756–66. DOI: 10.1002/jemt.22227. PMID: 23650099.

18. Skandalakis JE, Gray SW. 1994. Embryology for surgeons: the embryological basis for the treatment of congenital abnormalities. 2nd ed. Williams & Wilkins;Baltimore: p. 217. DOI: 10.1002/jemt.22227.
19. Lemke C, Biedermann U. 2021; A persistent vitelline artery in an adult. Case report and review of literature. Transl Res Anat. 22:100080. DOI: 10.1016/j.tria.2020.100080.

20. Zovein AC, Turlo KA, Ponec RM, Lynch MR, Chen KC, Hofmann JJ, Cox TC, Gasson JC, Iruela-Arispe ML. 2010; Vascular remodeling of the vitelline artery initiates extravascular emergence of hematopoietic clusters. Blood. 116:3435–44. DOI: 10.1182/blood-2010-04-279497. PMID: 20699440. PMCID: PMC8127023.

21. Wright JR Jr. 2019; Prevalence, morphology, embryogenesis, and diagnostic utility of umbilical cord vitelline vascular remnants. Pediatr Dev Pathol. 22:279–87. DOI: 10.1177/1093526618811734. PMID: 30541420.

22. De Guzman JK, Yu W, Horn C, Brundler MA, Wright JR Jr. 2021; Characterization of vitelline vessel remnant circulation in the umbilical cord. Placenta. 111:97–104. DOI: 10.1016/j.placenta.2021.06.009. PMID: 34225217.

23. Jaiman S, Nalluri HB. 2013; Abnormal continuation of umbilical vein into extra-hepatic portal vein: report of three cases. Congenit Anom (Kyoto). 53:170–5. DOI: 10.1111/cga.12020. PMID: 24712478.

24. Martin BF, Tudor RG. 1980; The umbilical and paraumbilical veins of man. J Anat. 130(Pt 2):305–22. PMID: 7400038. PMCID: PMC1233134.
25. Ibukuro K, Fukuda H, Tobe K, Akita K, Takeguchi T. 2016; The vascular anatomy of the ligaments of the liver: gross anatomy, imaging and clinical applications. Br J Radiol. 89:20150925. DOI: 10.1259/bjr.20150925. PMID: 27163944. PMCID: PMC5124872.

Fig. 1
Yolk stalk containing the vitelline vessels and yolk sac remnant outside of the umbilical cord (7.5 mm CRL). H&E staining. Horizontal sections. Panel (A) displays the most superior plane or the most upstream portion of the umbilical cord in the figure. The yolk stalk is separated from the umbilical cord. The yolk stalk contains the VA and VV as well as a vesicle corresponding to the yolk sac remnant. In the inferior or downstream side of the yolk stalk, the mesentery appears in the yolk stalk (D, E) and, subsequently, the VA and VV continue to a site near the IL (F, G). In more inferior or downstream portions, the artery increases in diameter, while the vein is still thin and separated from the mesentery (G–I). The SMV develops secondarily as a cleft of tissues (H, J). Panel (K) is a diagram showing topographical anatomy of the vitelline vessels and yolk sac. The vitelline vein runs outside of the mesentery (star). Panels (A–D) and (E–J) were prepared at the same magnification, respectively (all scale bars, 0.1 mm). CRL, crown-rump length; VA, vitelline artery; VV, vitelline vein; UA, umbilical artery; UV, umbilical vein; SMA, superior mesenteric artery and its branches; GB, gall bladder; SMV, superior mesenteric vein and its tributaries; PV, portal vein; AO, aorta; IL, ileum; JE, jejunum.
Fig. 2
Yolk sac remnant seen in sagittal sections (8 mm CRL). Panels (A–C) (H&E staining) display an embryo of 8 mm CRL, and (D, E) (silver impregnation) exhibits another embryo of 8 mm CRL. Sagittal sections demonstrate the yolk sac remnant immediately below the liver and heart in the two embryos. The yolk stalk appears to contain the vitelline vessels as well as a vacuole-like yolk sac (D, E). In both specimens, a combination of the sac, artery and vein appears to extend from the intra-embryonic coelom to the extra-embryonic coelom. All panels were prepared at the same magnification (scale bar in E, 1 mm). CRL, crown-rump length; AO, aorta; UV, umbilical vein; SMA, superior mesenteric artery and its branches; UA, umbilical artery; VA, vitelline artery; VV, vitelline vein; IL, ileum.
Fig. 3
Yolk stalk containing the vitelline vessels and yolk sac remnant inside of the umbilical cord (9 mm CRL). H&E staining. Horizontal sections. Panel (A) (or G) displays the most superior (or inferior) plane in the figure. The umbilical cord contains the yolk stalk (A–C). The yolk stalk contains the VA and VV as well as a vesicle corresponding to the yolk sac remnant (A, B). In the inferior or downstream portion of the yolk stalk near the IL, the vitelline vein detaches from the stalk (C) and runs freely in the extra- and intra-embryonic coelom (D–F). The free vein attaches to the duodenum to connect with the PV (F). The SMV develops secondarily as a cleft of tissues (G). All panels were prepared at the same magnification (scale bar in A, 1 mm). CRL, crown-rump length; UA, umbilical artery; UV, umbilical vein; VV, vitelline vein; VA, vitelline artery; PV, portal vein; SMA, superior mesenteric artery and its branches; RUV, right umbilical vein; CA, celiac artery; DP, dorsal pancreas; LUV, left umbilical vein; AO, aorta; SMV, superior mesenteric vein and its tributaries; IL, ileum; JE, jejunum.
Fig. 4
Yolk stalk containing the vitelline vessels and yolk sac remnant inside of the umbilical cord (10 mm CRL). Silver impregnation. Horizontal sections. Panel (A) displays the most inferior plane or the most upstream portion of the umbilical cord in the figure. The yolk stalk is contained in the umbilical cord (A–G). The yolk stalk contains the VA and VV as well as a large vesicle corresponding to the yolk sac remnant (A–D). The yolk sac appears to open to the VV (arrow in E). In the superior or downstream portion when the mesentery appears in the yolk stalk, the VV detaches from the stalk (F) and runs freely in the extra- and intra-embryonic coelom (G–I). The free vein attaches to the stomach pylorus (J) to connect the PV (K). The artery is thick along the almost entire course toward the superior mesenteric artery near the duodenum. Panel (L) is a diagram showing topographical anatomy of the vitelline vessels and yolk sac: the sac appears to open to the vein (arrow). The VV runs outside of the mesentery (star). All panels were prepared at the same magnification (scale bar in A, 1 mm). CRL, crown-rump length; UA, umbilical artery; UV, umbilical vein; VA, vitelline artery; VV, vitelline vein; AO, aorta; SMA, superior mesenteric artery and its branches; SMV, superior mesenteric vein and its tributaries; PCV, posterior cardinal vein; PV, portal vein; P, pancreas; LUA, left umbilical artery; RUA, right umbilical artery; IL, ileum.

Fig. 5
Extra-embryonic long courses of the vitelline vessels without a yolk sac remnant (11 mm CRL). H&E staining. Horizontal sections. Panel (A) displays the most inferior plane in the figure, while (H) exhibits the upstream portion of the umbilical cord containing long courses of the vitelline vessels (VA, VV). Panels (B–G) are shown at higher magnification than (A, H). The VV is dilated along the free intra-embryonic course (B–D). The superior mesenteric vein is also thick in the mesentery but the luminal surface is not smooth (B, C). In the upstream side of the IL, the vitelline vessels take a long extra-embryonic course without a definite yolk sac remnant (F, G). Thus, the vessels appear to run freely without a yolk stalk. Panels (A, H) (or B–G) were prepared at the same magnification (scale bars in A and B, 1 mm). CRL, crown-rump length; UA, umbilical artery; UV, umbilical vein; VA, vitelline artery; VV, vitelline vein; PCV, posterior cardinal vein; AO, aorta; SMA, superior mesenteric artery and its branches; SMV, superior mesenteric vein and its tributaries; RUV, right umbilical vein; CA, celiac artery; GB, gall bladder; IL, ileum; JE, jejunum.
Fig. 6
Extra-embryonic long courses of the vitelline vessels without a yolk sac remnant (13 mm CRL). H&E staining. Horizontal sections. Panel (A) displays the most superior plane in the figure, while (F) exhibits the upstream portion of the umbilical cord. In the extra-embryonic coelom, not only the VV (A–E) but also the VA (D, E) run freely without the yolk stalk and sac. All panels were prepared at the same magnification (scale bars in A and B, 1 mm). CRL, crown-rump length; LUV, left umbilical vein; RUV, right umbilical vein; VV, vitelline vein; VA, vitelline artery; LUA, left umbilical artery; RUA, right umbilical artery; SMA, superior mesenteric artery and its branches; IL, ileum; JE, jejunum.

Fig. 7
Yolk sac remnant near the future umbilicus identified in sagittal sections (20 mm CRL). H&E staining. Sagittal sections of the right half of the abdomen. Panel (A) is a diagram showing topographical anatomy of the VV, yolk sac and superior mesenteric vessels (SMA, SMV): the sac appears to communicate the SMV. The vitelline vein runs outside of the mesentery (star). Panel (B) displays the most upstream portion of the umbilical cord in the figure. Panel (B) (or G) is lateral (medial). A thin VV remnant takes a long course free from the mesentery in the extra-embryonic- (B–D) and intra-embryonic coelom (E, F). The vein ends near the duodenum (G). The SMV is dilated in the mesentery (D). A candidate of the yolk sac remnant (E–G) opens to the vein (arrows in F). All panels were prepared at the same magnification (scale bar in B, 1 mm). CRL, crown-rump length; SMA, superior mesenteric artery and its branches; SMV, superior mesenteric vein and its tributaries; VV, vitelline vein; UA, umbilical artery; GB, gall bladder; IL, ileum; JE, jejunum.

Fig. 8
Schematic representation of a regression process of the vitelline vessels and yolk sac at 5–6 weeks. Panel (A) is a left side view of a 5-week-old embryo, while (B) exhibits the intestinal loop in the herniation sac at 6 weeks and later. In (A), the yolk sac remnant and vitelline vessels extend distally to the apex of the midgut loop. The umbilical cord bundles the umbilical vessels, the yolk sac, and the allantois. The regressing yolk sac is a vacuole sandwiched by the VA and VV. In the proximal or posterior side of the apex of the midgut loop, the artery changes the name into the SMA. In contrast to the long free course of the VV, the SMV develops secondarily in the mesentery. In (B), in contrast to the lost VA, a remnant of the VV still runs through the extra-embryonic celom or herniation sac. However, usually, the umbilical cord does not contain the VV remnant. The yolk sac almost always disappears. The allantois is not shown in (B). SMA, superior mesenteric artery and its branches; SMV, superior mesenteric vein and its tributaries; UA, umbilical artery; UV, umbilical vein; VA, vitelline artery; VV, vitelline vein.
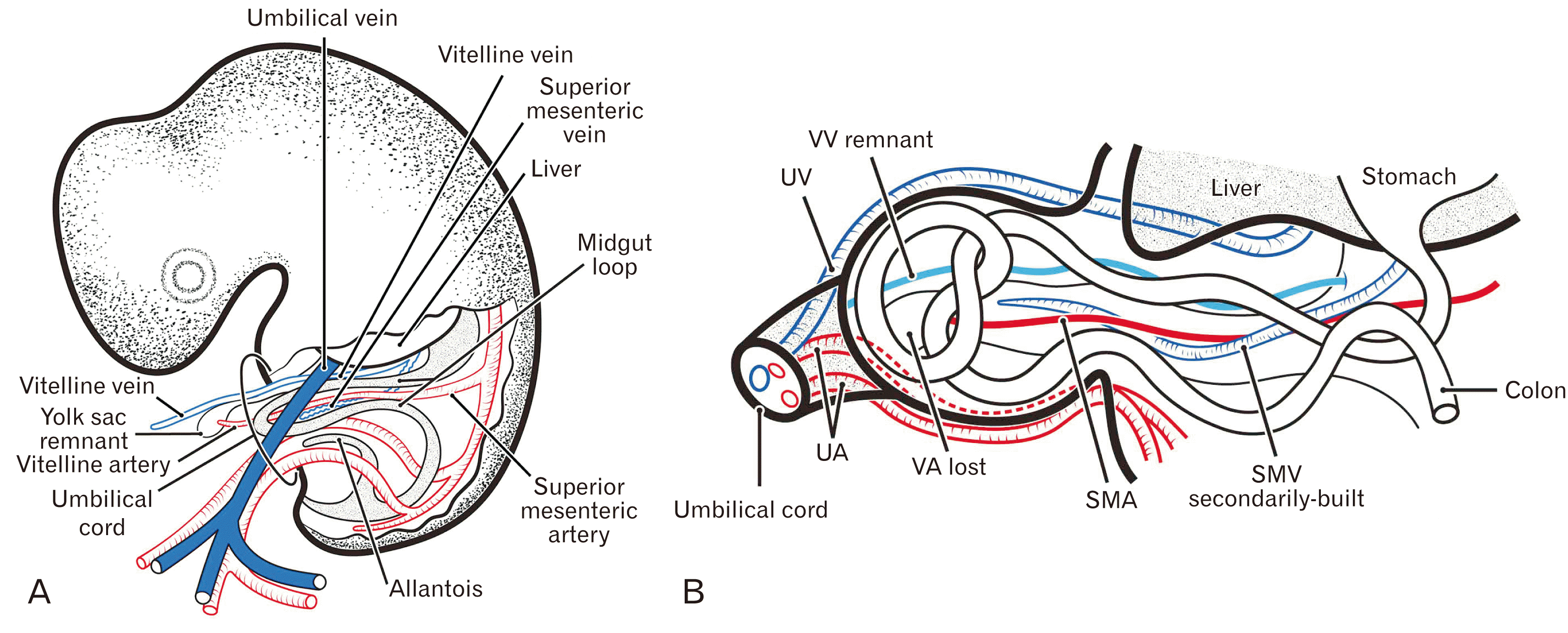
Table 1
Age- or method-dependent difference in anatomy of the yolk sac remnanat and vitelline vessels
| Specimen A–T(Figures shown) | VA distal to themidgut apex | Yolk sac remnant | Free VV into the PV near the duodenumb | |
|---|---|---|---|---|
| A. | 6 mm sag | Uncleara | + | + |
| B. | 7.5 mm hr (Fig. 1) | + | + | + |
| C. | 8 mm sag (Fig. 2) | Uncleara | + | + |
| D. | 8 mm sag (Fig. 2) | Uncleara | + | + |
| E. | 8 mm hr | + | + | + |
| F. | 9 mm hr (Fig. 3) | + | + | + |
| G. | 9 mm sag | Uncleara | + | + |
| H. | 10 mm hr | + | + | + |
| I. | 10 mm hr (Fig. 4) | + | + | + |
| J. | 11 mm hr (Fig. 5) | + (1.2 mm) | – | + |
| K. | 12 mm hr | + | – | – |
| L. | 12 mm hr | + | + | + |
| M. | 13 mm hr (Fig. 6) | + (short) | – | + |
| N. | 15 mm hr | – | – | + |
| O. | 15 mm hr | + (short) | – | – |
| P. | 16 mm hr | – | + | + |
| Q. | 16 mm sag | – | – | – |
| R. | 18 mm hr | – | – | – |
| S. | 18 mm hr | – | + | + |
| T. | 20 mm sag (Fig. 7) | – | + | + |
VA, vitelline artery; VV, vitelline vein; PV, portal vein; sag, sagittal sections; hr, horizontal sections; +, find; –, not find.
aSagittal sections often made a distal continuation of the VA unclear possibly due to tissue injury during the histological procedure. bA fragment or part of the VV free from the mesentery was usually found in the intra-embryonic coelom, but sometimes in larger specimens, we did not find the terminal to the PV or mesenteric vein near the duodenum.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download