Abstract
Vascular anomalies are a serendipitous finding during surgeries and diagnostic angiography. Such variations are frequently encountered in the abdominal region. These anomalies are usually asymptomatic but the presence of hepatic arterial variations may lead to injuries of the liver during surgery. The present study was conducted on 35 adult embalmed cadavers, 31 males, 4 females from August 2015 to December 2021 in the Department of Anatomy, Amrita School of Medicine, Amrita Institute of Medical Sciences, Kochi. In this study of 35 cadavers, we present 3 variants: an accessory right hepatic artery, replaced common hepatic artery, replaced common hepatic artery anastomosis with accessory left hepatic artery and an arc of Buhler. One of our variants has not yielded a precedent in literature search. We have compared these variants with Michels and Hiatt classification. It is known that different variants arise at distinct stages of embryonic development. As specialists in anatomy, we have tried to correlate the variants in our study with their embryological origins.
Vascular anomalies are a serendipitous finding during surgeries and diagnostic angiography. Such variations are frequently encountered in the abdominal region. In the era of transplantations, liver transplants are now common procedure and therefore mapping its vascularity is important to the gastro surgeon. While the hepatic artery provides only 30% of blood flow for the liver, they are important for the vascularity of the bile duct [1].
In the classical description, the coeliac trunk (CT) trifurcates into left gastric artery (LGA), splenic artery (SA), and common hepatic artery (CHA). The first documented work on the CT was by Von Haller [2] in 1756, where he described its normal and aberrant branches. Within the ambit of aberrant arteries are accessory and replaced arteries. A vessel which supplies a liver lobe additional to its usual vessel is defined as an accessory hepatic artery. A vessel that supplies a liver lobe in lieu of the normal is a replaced hepatic artery [3]. These anomalies are usually asymptomatic but the presence of hepatic arterial variations may lead to injuries of the liver during surgery. The presence of these arteries can be explained embryologically. Michels [3] in 1966 studied 200 cadavers and developed a classification with 10 CT subsets. Type I: normal pattern, Type II: replaced left hepatic artery (LHA) from the LGA, Type III: replaced right hepatic artery (RHA) branch of superior mesenteric artery (SMA), Type IV: replaced LHA & replaced RHA, Type V: accessory left hepatic artery (aLHA) branch of LGA, Type VI: accessory right hepatic artery (aRHA) branch of SMA, Type VII: aRHA & LHA, Type VIII: aLHA & replaced RHA, aRHA & replaced LHA, Type IX: CHA branch of SMA, Type X: CHA branch of LGA. Hiatt [4] in 1994 reorganized these into 6 categories. Type I: normal pattern, Type II: replaced LHA from the LGA & aLHA branch of LGA, Type III: replaced RHA branch of SMA & aRHA branch of SMA, Type IV: replaced LHA & replaced RHA, aLHA & aRHA, aLHA & replaced RHA, aRHA & replaced LHA, Type V: aLHA branch of LGA, Type VI: aRHA branch of SMA. Hiatt disregarded the concept of segregating accessory and replaced arteries. He based the origin of the artery as the criteria and put together in it, both accessory and replaced arteries. Thereby, he consolidated the 10 types in Michels classification into 6 types.
In this study of 35 cadavers, we present 3 variants (Table 1). We have compared these variants with Michels and Hiatt classification. One of our variants has not yielded a precedent in literature search.
The present study was conducted on 35 adult formalin embalmed cadavers of which 31 were males, 4 were females and belonged to the age group 55 to 80 years. The study was carried out from August 2015 to December 2021 in the Department of Anatomy, Amrita School of Medicine, Amrita Institute of Medical Sciences, Kochi.
These findings were obtained during routine dissection study for first MBBS students and hence no separate institutional ethical committee approval was sought.
The abdomen was dissected following standard procedure described in Cunningham’s Dissection Manual Vol-II. The abdomen was opened. The stomach was identified. The lesser omentum was traced. Its attachments along the lesser curvature and the porta hepatis were defined. All arteries proceeding to the liver were retraced to the parent artery—CT/SMA. A schema was drawn in each case. Variations in origin and course of hepatic arteries as well as its relations with biliary duct were studied and photographs taken. All accessory vessels in this study are prefixed with ‘a’ and all replaced vessels with ‘r.’
Out of 35 cadavers studied, variations in origin and course of hepatic artery were observed in 3 of the 35 specimens, amounting to 8.5% (3/35), each variant being 2.86%. The remaining 32 cadavers showed classical origin and course of hepatic arteries (32/35=91.4%).
Case 1: A male cadaver with aRHA (Fig. 1). The CT measured 3 cm and was directed upwards and to the right, where it trifurcated. The LGA arose from its anterior wall and coursed upwards and to the left. The SA ran upwards for 1 cm and turned at right angles to the left to run a tortuous course towards the spleen. The CHA made a course slightly upwards and to the right and gave off the gastroduodenal artery (GDA) 3 cm from its origin and continued as the HA proper making its entry into porta hepatis, passing through the lesser omentum. The SMA was noted 1 cm below the CT. About 1.5 cm from its origin an artery was observed going towards the right end of the porta hepatis, qualifying as an aRHA as the CHA was present. It was found passing behind the superior mesenteric vein (SMV), the head of pancreas, first part of duodenum and ascends right to portal vein entering the lesser omentum in company with the bile duct and the portal vein. The right free margin of the lesser omentum was greatly increased in thickness due to the presence of aRHA reducing the aperture of the foramen of Winslow, admitting only the tip of the little finger.
Case 2: A female cadaver with replaced common hepatic artery (rCHA) from SMA (Fig. 2). The CT was noted to be about 2 cm and directed upwards and to the left. It bifurcated into a small artery which was traced along the lesser curvature of the stomach and hence designated as the LGA. The other artery was found to have an initial spiral course in the upward direction and then it turned abruptly to the left side towards the spleen. It had the tortuosity normally stated for a SA. We traced the SMA and found rCHA arising 2 cm from its origin and coursing its way to the porta hepatis. The GDA arose 4 cm from its origin was found running downwards. At the porta hepatis it gave off the right and left hepatic branches, while the main artery took a circuitous course and was traced along the lower part of lesser curvature as right gastric artery (RGA).
Case 3: A male cadaver with rCHA and aLHA (Fig. 3). The CT bifurcated. The artery that was positioned upwards and to the left was traced along the lesser curvature of the stomach and was therefore designated as the LGA. An artery arising from the LGA was seen coursing towards porta hepatis. The SA was identified as a tortuous vessel travelling to the left side. Both these vessels had smaller caliber than normal. The SMA was identified; 1 cm from its origin two arteries were seen from its right and left walls below the lower border of pancreas, the left being larger than the right, both merged to form the rCHA at the level of middle of head of pancreas anteriorly. The right and left divisions formed a loop around the SMV before its merger, with the left division of the aberrant rCHA lying anteriorly and the right division of the aberrant rCHA lying posterior to it. After the merger, the rCHA proceeded upwards along with bile duct and portal vein towards the right end of porta hepatis which then branched into RHA and LHA. Therefore, the artery from the LGA lying in the left part of porta hepatis qualifies to be aLHA. This artery met the rCHA forming a loop within porta hepatis.
About 1 cm proximal to the union of two divisions to form the rCHA an artery was traced from the anterior (left division) rCHA and was identified as the RGA as it ran along the lesser curvature of the stomach when traced from the pylorus. Another artery was traced from the posterior (right division) of the rCHA and was identified as the GDA.
A ‘y’ shaped segment of artery was noted retro pancreatic, between the anterior (left division) of the rCHA and SA, thereby forming a connection between the CT and SMA or an arc of Buhler. In addition to this the anterior (left division) of the rCHA was seen to give off branches having courses similar to the right colic artery and middle colic artery.
Variations of arteries irrigating solid and hollow viscera of the abdomen, especially the liver have been highlighted by anatomists and radiologists alike due to their significance in surgeries involving viscerae [5-7]. The anatomy and variations of hepatic artery have been described by several authors like Von Haller [2] in 1756, Tiedemann [8] in 1822, Flint [9] in 1923, Adachi [10] in 1928, and Michels [11] in 1955. While Hallers work was exclusive to the celiac trunk, Tiedemann’s work was unrestricted to multiple vascular anomalies of human body. Both Adachi and Michel studied hepatic artery and arrived at various subgroups based on the origin of the vessels. Flint in his seminal work on arterial anomalies of the right hepatic, cystic and GDA included their relation to the bile duct.
Various literature records set the prevalence of anatomical variants in the range of 20% to 50% [12, 13]. It is known that different variants arise at distinct stages of embryonic development.
The CHA in adults is intermediate in size between LGA and SA, although in the foetus and early postnatal life it is the largest branch of the CT. After arising from CT, it passes anteriorly and laterally behind epiploic foramen beyond the origin of GDA it is termed hepatic artery. At the porta hepatis, it divides into right and left branches [14]. This classical description was seen in 32 (91.43%) of the cases in the present study. Michel and Hiatt studies which are the gold index of hepatic artery variants reported classical description of 55.61% and 75%–77% respectively [3, 4].
The higher percentage in the present study can be reasoned to be definitely due to the smaller sample size and questionably due to ethinicity. Other Indian studies also report higher numbers of normal pattern than in Caucasian studies [15].
The course of the 3 variants can be embryologically correlated to either persistence of a foetal variant or disappearance of a vessel that should have persisted. The type VII, VIII, IX, and X of the Michel classification and its equivalent in the Hiatt classification have been rarely described in literature, while many cases of RHA arising from SMA has been documented.
Based on ethinicity, the present study can be juxtaposed with that undertaken by Ghosh [15] on 125 cadavers. In methodology his study is more extensive than this study, in that, he traced the hepatic artery further into the liver. His study revealed 123 trifurcations and 2 bifurcations of the CT. In the present study we have noted 33 trifurcations and 2 bifurcations. In absolute numbers the two studies have 2 bifurcated CTs in the splenogastric pattern. The 2 bifurcations in Ghosh’s study tabulates to 1.6%, whereas in the present study it stands at 5.7%, which can be attributed to the smaller sample size studied. Another point of difference between the two studies is the presence of replaced and accessory arteries unlike Ghosh’s study where only accessory arteries were noted.
In case 1 variant of our study, we observed the aRHA to be arising from the SMA, this can be categorised as type VI of the Michel classification and type III of the Hiatt’s classification (Table 1). Similar cases were reported by various authors [16-18]. The results in the present study of this variant was in keeping with the findings of Michels (1.5%–7%) [3], Hiatt (10.6%) [4], Farghadani et al. (0.5%) [19], Fonseca-Neto et al. (0.4%) [20], Cherian et al. (4%) [21], Dandekar et al. (3.4%) [22], Zhang et al. (5.5%) [23], Zaki et al. (2.8%) [24].
In case 2 variant of the present study, we observed the rCHA to be arising from the SMA. At the porta hepatis it gave off the right and left hepatic branches, while the main artery took a circuitous course and was traced along the lower part of lesser curvature as RGA. Upto the point in the porta hepatis this artery has semblance to type IX of Michel classification and type V of Hiatt classification but the presence of RGA will not essentially allow it to fall into the stated subtypes. Some authors have referred this variant as hepatomesenteric trunk [6, 25, 26]. This extended pattern has not been mentioned in any study in our literature search.
Prevalence of rCHA has been reported in literature to range from 0.4 to 4.5 [4, 27]. Replaced CHA to be arising from the SMA were reported by various authors [6, 28]. The results of the present study compare well with the findings of Michels (2%–4.5%) [3], Hiatt (1.5%) [4], Zagyapan et al. (6.6%) [29], Kadir et al. (5%) [30], Woods and Traverso (2.5%) [31], Sethy et al. (4%) [32], Zhang et al. (2.8%) [23], Zaki et al. (0.4%) [24].
Ha et al. [33] in their study observed that the rCHA does not penetrate the pancreatic parenchyma and crosses the dorsal aspect of portal vein and SMV. In the present study the rCHA is retro pancreatic but lies ventral to portal vein.
In case 3 variant of our study, rCHA a branch of SMA, anastomosis with aLHA derived from LGA. This rCHA branch of SMA giving off GDA and RHA and LHA at its termination along with an aLHA from the LGA therefore is a new subset hitherto unmentioned in any classification of the hepatic artery. aLHA from the LGA was reported by various authors [2, 17-19, 34] .
The course of HA in case 2 and case 3 do not fall into category of any classification to the best of our knowledge (Table 1).
The variant vessels can be explained embryologically. The primitive liver is supplied by three embryonic hepatic arteries named left, right, and common hepatic arteries. The LHA arises from the LGA and RHA from the SMA and they undergo regression whereas CHA persists. If the arteries that must undergo regression persist aberrant hepatic artery results.
During foetal life the dorsal aorta and ventral aorta are anastamosed by several vitelline arteries of significance. The vitelline arteries, 10, 11, 12 form the CT with the disappearance of the ventral anastomotic segment and then give rise to LGA, CHA, and SA. The 13th gives rise to SMA and in the process the anastomotic segment between it and 12th disappears and that is how the coeliac and SMA become 2 entities [35].
In case 1: 10, 11, 12 vitelline arteries have developed normally and develop as the normal offshoots of coeliac axis. From the 13th vitelline artery, the foetal RHA persists therefore there is normal development of CHA with an accessory RHA.
In case 2: The 11th vitelline artery is absent and the CT has the outgrowth of 10th and 12th vitelline arteries giving off the LG and SA, respectively. The 13th vitelline artery with its offshoot, the foetal RHA persists to irrigate the liver and hence is a replaced aberrant artery.
In case 3: the 11th vitelline artery has disappeared. The foetal LHA from the 10th vitelline artery persists as the LHA. The foetal RHA from the 13th vitelline artery persists but with two formative divisions. In addition, the ventral anastomosis between the 12th and 13th vitelline arteries persists, forming the arc of Buhler. We are unable to embryologically correlate the split origin of rCHA as well as the aberrant arc of Buhler (Fig. 4).
Aberrant hepatic artery arising from LGA need to be recognised during mobilization of stomach in surgeries related to hiatal hernia and gastrectomy [34]. The rCHA becomes important during pancreas and duodenal surgeries. Accidental ligation of aberrant hepatic artery may lead to liver necrosis and death. Interventional radiologists performing hepatic arterial embolization should be familiar with permutations and combinations of hepatic artery as failure to recognise these arteries may lead to incomplete treatment modality.
The CT and its branches are significant in surgeries such as gastric resection, partial hepatectomy, operations performed near the gastro hepatic ligament, including esophagogastrectomy, gastric bypass, and anti-reflux procedures. Therefore, the knowledge of the existence of either accessory or replaced is important as they may be potential bleeding sites, or even the only source of vascularity.
Notes
References
1. Blumgart LH, Belghiti J. 2007. Surgery of the liver, biliary tract, and pancreas. 4th ed. Saunders Elsevier;Philadelphia: DOI: 10.1016/B978-1-4160-3256-4.50009-0.
2. Von Haller A. 1756. Icones anatomicae quibus praecipuae aliquae partes corporis humani delineatae proponuntur et arteriarum potissimum historia continetur. Vandenhoeck;Gottingae: p. 270. Latin.
3. Michels NA. 1966; Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg. 112:337–47. DOI: 10.1016/0002-9610(66)90201-7. PMID: 5917302.

4. Hiatt JR, Gabbay J, Busuttil RW. 1994; Surgical anatomy of the hepatic arteries in 1000 cases. Ann Surg. 220:50–2. DOI: 10.1097/00000658-199407000-00008. PMID: 8024358. PMCID: PMC1234286.

5. Koshariya M, Khare V, Songra MC, Shukla S, Gupta A. 2021; Anomalous anatomical variations of coeliac trunk: a cadaveric study. Cureus. 13:e19108. DOI: 10.7759/cureus.19108. PMID: 34868759. PMCID: PMC8627549.

6. Noussios G, Dimitriou I, Chatzis I, Katsourakis A. 2017; The main anatomic variations of the hepatic artery and their importance in surgical practice: review of the literature. J Clin Med Res. 9:248–52. DOI: 10.14740/jocmr2902w. PMID: 28270883. PMCID: PMC5330766.

7. Malki Y, Lazaar H, Bouhout T, Serji B, El Harroudi T. 2021; Replaced common hepatic artery arising from the superior mesenteric artery discovered over a pancreaticoduodenectomy: a case report. Clin Surg. 6:3247.
8. Tiedemann F. 1822. Tabularum arteriarum corporis humani. Muller;Carlsruhae: p. 1–250. Latin.
9. Flint ER. 1923; Abnormalities of the right hepatic, cystic, and gastroduodenal arteries, and of the bile-ducts. Br J Surg. 10:509–19. DOI: 10.1002/bjs.1800104011.

10. Adachi B. 1928. Das Arteriensystem der Japaner. Vol.2:Kaiserlich-Japanischen Universitat;Kyoto: German. DOI: 10.1002/bjs.1800104011.
11. Michels NA. 1955. Blood supply and anatomy of the upper abdominal organs with a descriptive atlas. Lippincott;Philadelphia: DOI: 10.1002/bjs.1800104011.
12. Andraus W, Haddad LB, Ducatti L, Martino RB, Santos VR, D'Albuquerque LA. 2013; Artery reconstruction in liver transplantation: the best reconstruction of right hepatic artery variation. Arq Bras Cir Dig. 26:62–5. DOI: 10.1590/S0102-67202013000100014. PMID: 23702874.
13. Sebben GA, Rocha SL, Sebben MA, Parussolo Filho PR, Gonçalves BH. 2013; Variations of hepatic artery: anatomical study on cadavers. Rev Col Bras Cir. 40:221–6. DOI: 10.1590/S0100-69912013000300010. PMID: 23912370.
14. Standring S. 2008. Gray's Anatomy: the anatomical basis of clinical practice. 40th ed. Churchill Livingstone;Edinburgh: p. 1072–4. p. 1116–7. p. 1130p. 1169–70.
15. Ghosh SK. 2014; Variations in the origin of middle hepatic artery: a cadaveric study and implications for living donor liver transplantation. Anat Cell Biol. 47:188–95. DOI: 10.5115/acb.2014.47.3.188. PMID: 25276478. PMCID: PMC4178194.

16. Kumar N, Swamy RS, Shetty P, Nayak SB, Patil J, Shetty SD, Guru A. 2014; Accessory right hepatic artery compensating rudimentary right branch of hepatic artery proper - a case report. Int J Health Sci Res. 4:331–4.
17. Eid N, Allouh M, Ito Y, Taniguchi K, Adeghate E. 2021; Jul. 5. Accessory right hepatic artery and aberrant bile duct in the hepatocystic triangle: a rare case with clinical implications. Folia Morphol (Warsz). [Epub]. https://doi.org/10.5603/FM.a2021.0065. DOI: 10.5603/FM.a2021.0065. PMID: 34219214.

18. Li X, Zhang X, Lu Q, Li A, Lin J, Fan H, Tang R. 2019; An accessory right hepatic artery derived from the superior mesenteric artery for anterior right liver lobe supply: a case report. Surg Radiol Anat. 41:969–71. DOI: 10.1007/s00276-018-2173-3. PMID: 30580394. PMCID: PMC6620246.

19. Farghadani M, Momeni M, Hekmatnia A, Momeni F, Baradaran Mahdavi MM. 2016; Anatomical variation of celiac axis, superior mesenteric artery, and hepatic artery: evaluation with multidetector computed tomography angiography. J Res Med Sci. 21:129. DOI: 10.4103/1735-1995.196611. PMID: 28331515. PMCID: PMC5348823.

20. Fonseca-Neto OCLD, Lima HCS, Rabelo P, Melo PSV, Amorim AG, Lacerda CM. 2017; Anatomic variations of hepatic artery: a study in 479 liver transplantations. Arq Bras Cir Dig. 30:35–7. DOI: 10.1590/0102-6720201700010010. PMID: 28489166. PMCID: PMC5424684.

21. Cherian PT, Hegab B, Oliff SP, Wigmore SJ. 2010; The management of an accessory or replaced right hepatic artery during multiorgan retrieval: results of an angiographic study. Liver Transpl. 16:742–7. DOI: 10.1002/lt.22075. PMID: 20517908.
22. Dandekar U, Dandekar K, Chavan S. 2015; Right hepatic artery: a cadaver investigation and its clinical significance. Anat Res Int. 2015:412595. DOI: 10.1155/2015/412595. PMID: 26788371. PMCID: PMC4695647.

23. Zhang W, Wang K, Liu S, Wang Y, Liu K, Meng L, Chen Q, Jia B, Liu Y. 2020; A single-center clinical study of hepatic artery variations in laparoscopic pancreaticoduodenectomy: a retrospective analysis of data from 218 cases. Medicine (Baltimore). 99:e20403. DOI: 10.1097/MD.0000000000020403. PMID: 32481341. PMCID: PMC7249910.
24. Zaki SM, Abdelmaksoud AHK, Khaled BEA, Abdel Kader IA. 2020; Anatomical variations of hepatic artery using the multidetector computed tomography angiography. Folia Morphol (Warsz). 79:247–54. DOI: 10.5603/FM.a2019.0090. PMID: 31436302.

25. Osawa T, Feng XY, Sasaki N, Nagato S, Matsumoto Y, Onodera M, Nara E, Fujimura A, Nozaka Y. 2004; Rare case of the inferior mesenteric artery and the common hepatic artery arising from the superior mesenteric artery. Clin Anat. 17:518–21. DOI: 10.1002/ca.10234. PMID: 15300873.

26. Kahraman G, Marur T, Tanyeli E, Yildirim M. 2001; Hepatomesenteric trunk. Surg Radiol Anat. 23:433–5. DOI: 10.1007/s00276-001-0433-z. PMID: 11963627.

27. Moszkowicz D, Peschaud F, El Hajjam M, Saiag P, Nordlinger B. 2011; Preservation of an intra-pancreatic hepatic artery during duodenopancreatectomy for melanoma metastasis. Surg Radiol Anat. 33:547–50. DOI: 10.1007/s00276-010-0770-x. PMID: 21221968.

28. Acebes-García F, Pinto-Fuentes P, Pacheco-Sánchez D, Choolani-Bhojwani E, Marcos-Santos P, Bueno-Cañones A, Veleda-Belanche S. 2021; Surgical significance of replaced common hepatic artery originating distally from the superior mesenteric artery during the whipple procedure. HPB. 23(Suppl 3):S910. DOI: 10.1016/j.hpb.2021.08.885.

29. Zagyapan R, Kürkçüoğlu A, Bayraktar A, Pelin C, Aytekin C. 2014; Anatomic variations of the celiac trunk and hepatic arterial system with digital subtraction angiography. Turk J Gastroenterol. 25(Suppl 1):104–9. DOI: 10.5152/tjg.2014.5406. PMID: 25910286.

30. Kadir S, Lundell C, Saeed M. Kadir S, Brothers MF, editors. 1991. Celiac, superior and inferior mesenteric arteries. Atlas of Normal and Variant Angiographic Anatomy. Saunders;Philadelphia: p. 297–308.
31. Woods MS, Traverso LW. 1993; Sparing a replaced common hepatic artery during pancreaticoduodenectomy. Am Surg. 59:719–21. PMID: 7902051.
32. Sethy S, Nayak GR, Agrawal D, Mohanty B, Biswal R. 2012; Anomalous origin of hepatic artery and its relevance in hepatobiliary surgeries. Int J Cur Res Rev. 4:143–8.
33. Ha HI, Kim MJ, Kim J, Park SY, Lee K, Jeon JY. 2016; Replaced common hepatic artery from the superior mesenteric artery: multidetector computed tomography (MDCT) classification focused on pancreatic penetration and the course of travel. Surg Radiol Anat. 38:655–62. DOI: 10.1007/s00276-016-1618-9. PMID: 26758052.

34. Tiwari S, Roopashree R, Padmavathi G, Varalakshmi KL, Sangeeta M. 2014; Study of aberrant left hepatic artery from left gastric artery and its clinical importance. Int J Cur Res Rev. 6:25–8.
35. Walker TG. 2009; Mesenteric vasculature and collateral pathways. Semin Intervent Radiol. 26:167–74. DOI: 10.1055/s-0029-1225663. PMID: 21326561. PMCID: PMC3036491.

Fig. 1
Accessory right hepatic artery (aRHA) arising from superior mesenteric artery (SMA). Liver lifted superiorly, stomach ligated and removed, pancreas reflected to the right, mobilised portal vein (PV) by cutting splenic vein (SV). Porta hepatis visualised with aRHA, greatly enlarged PV and bile duct (BD). LHA, left hepatic artery; RHA, right hepatic artery; CHA, common hepatic artery; GDA, gastroduodenal artery; RGA, right gastric artery; RGeA, right gastroepiploic artery; PHA, proper hepatic artery; AA, abdominal aorta; LGA, left gastric artery; CT, coeliac trunk; L, liver.
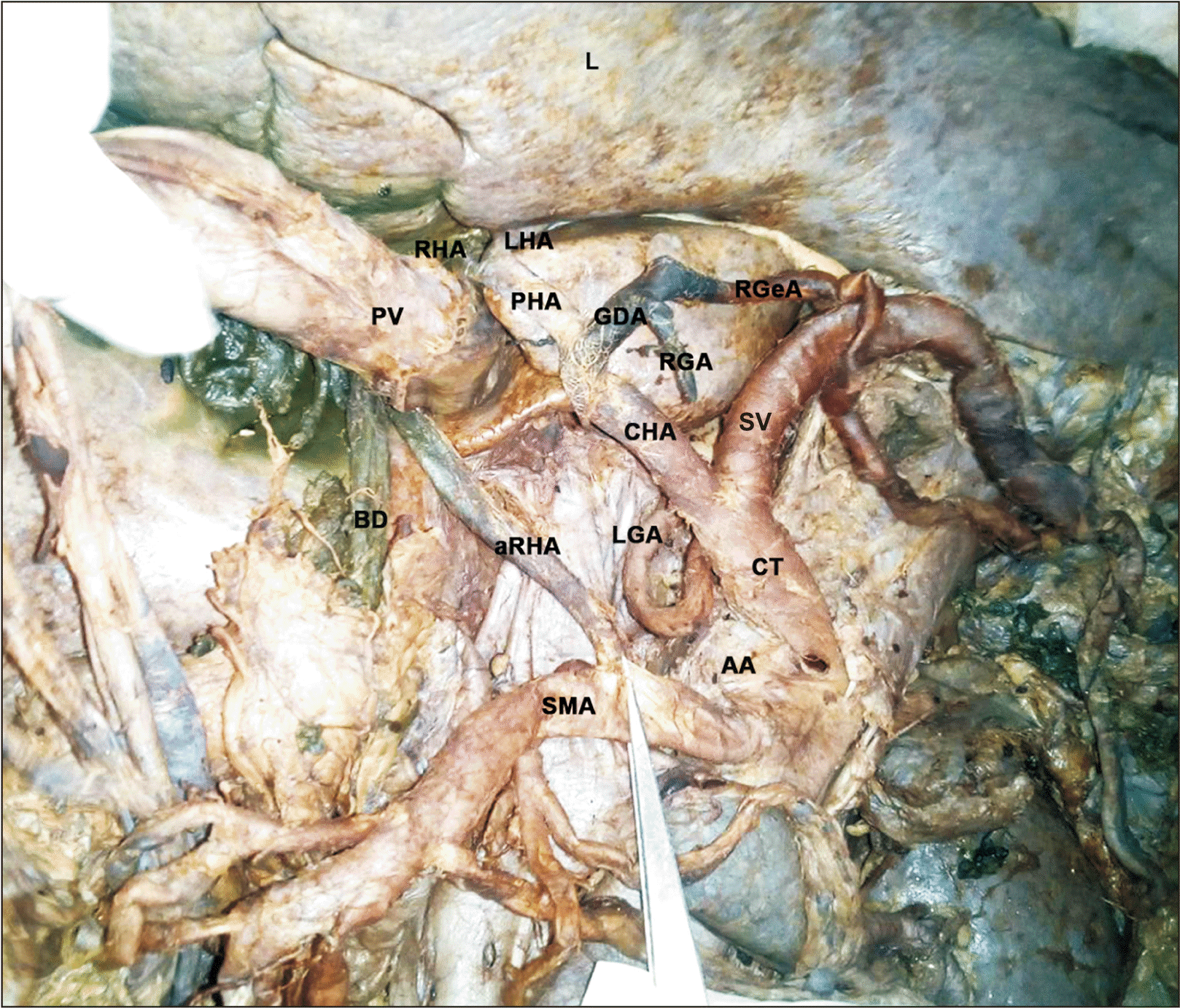
Fig. 2
rCHA arising from SMA. (A) Stomach cut at the pylorus, rCHA visualized. (B) Pancreas reflected to the right, rCHA visualized in its entirety, including its continuation as RGA along the lesser curvature. (C) Stomach reflected to the left, CT and its branches visualised. (D) rCHA and its relation to BD displayed. rCHA, replaced common hepatic artery; PHA, proper hepatic artery; GDA, gastroduodenal artery; SMA, superior mesenteric artery; PV, portal vein; RGA, right gastric artery; SA, splenic artery; LGA, left gastric artery; AA, abdominal aorta; CT, coeliac trunk; BD, bile duct; S, stomach; L, liver.
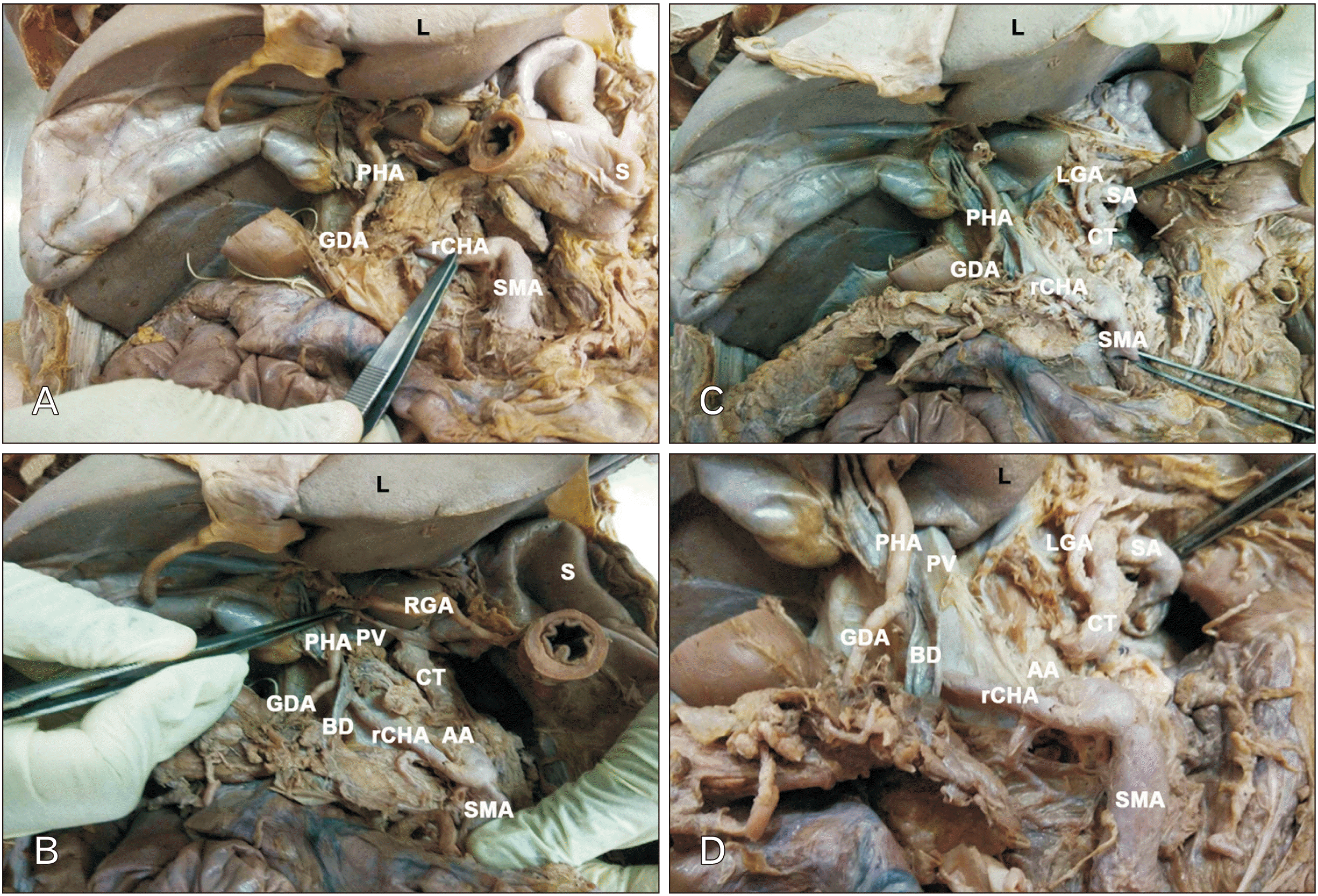
Fig. 3
rCHA arising from SMA with aberrant arc of Buhler. (A) Liver manoeuvred superiorly, lesser omentum cleared piecemeal, anastomosis between rCHA and aLGA seen. (B) Stomach resected, CT and its branches visualised. (C) Dual origin of rCHA and their merger around SMV seen. (D) Pancreas reflected to the right, arc of Buhler noted. rCHA, replaced common hepatic artery; aLGA, accessory left hepatic artery; LGA, left gastric artery; PV, portal vein; P, pancreas; BD, bile duct; GDA, gastroduodenal artery; RGA, right gastric artery; lrCHA, left division of rCHA; rrCHA, right division of rCHA; SMA, superior mesenteric artery; SMV, superior mesenteric vein; RCA, right colic artery; MCA, middle colic artery; SA, splenic artery; AA, abdominal aorta; CT, coeliac trunk; S, stomach; L, liver.
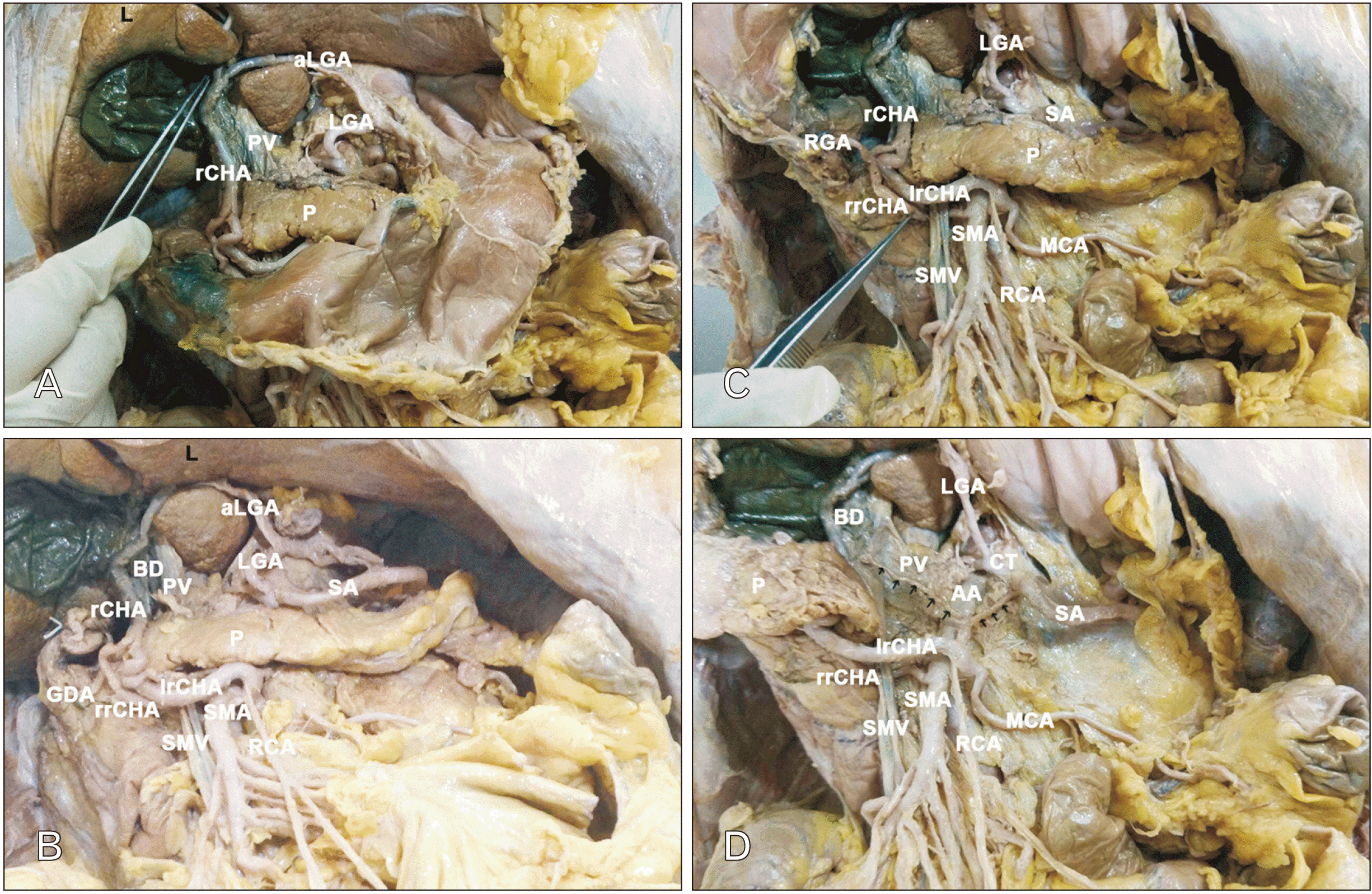
Fig. 4
Schematic representation of variants and embryological basis of variations observed in our study. (A) Primitive vasculature. (B) Embryological basis of normal development of coeliac trunk and superior mesenteric artery. (C) Accessory right hepatic artery arising from superior mesenteric artery. (D) Replaced common hepatic artery arising from superior mesenteric artery. (E) Replaced common hepatic artery arising from superior mesenteric artery anastomosis with accessory left hepatic artery from left gastric artery, arc of Buhler. VitA, vitelline artery; VA, ventral anastomosis; A, abdominal aorta; 1, abdominal aorta; 2, coeliac trunk; 3, superior mesenteric artery; 4, inferior mesenteric artery; 5, left gastric artery; 6, splenic artery; 7, common hepatic artery; 8, gastroduodenal artery; 9, right gastric artery; 10, proper hepatic artery; 11, right hepatic artery; 12, left hepatic artery; 13, middle colic artery; 14, right colic artery; r7, replaced common hepatic artery; lr7, left division of replaced common hepatic artery with arc of Buhler; rr7, right division of replaced common hepatic artery; a11, accessory right hepatic artery; a12, accessory left hepatic artery.
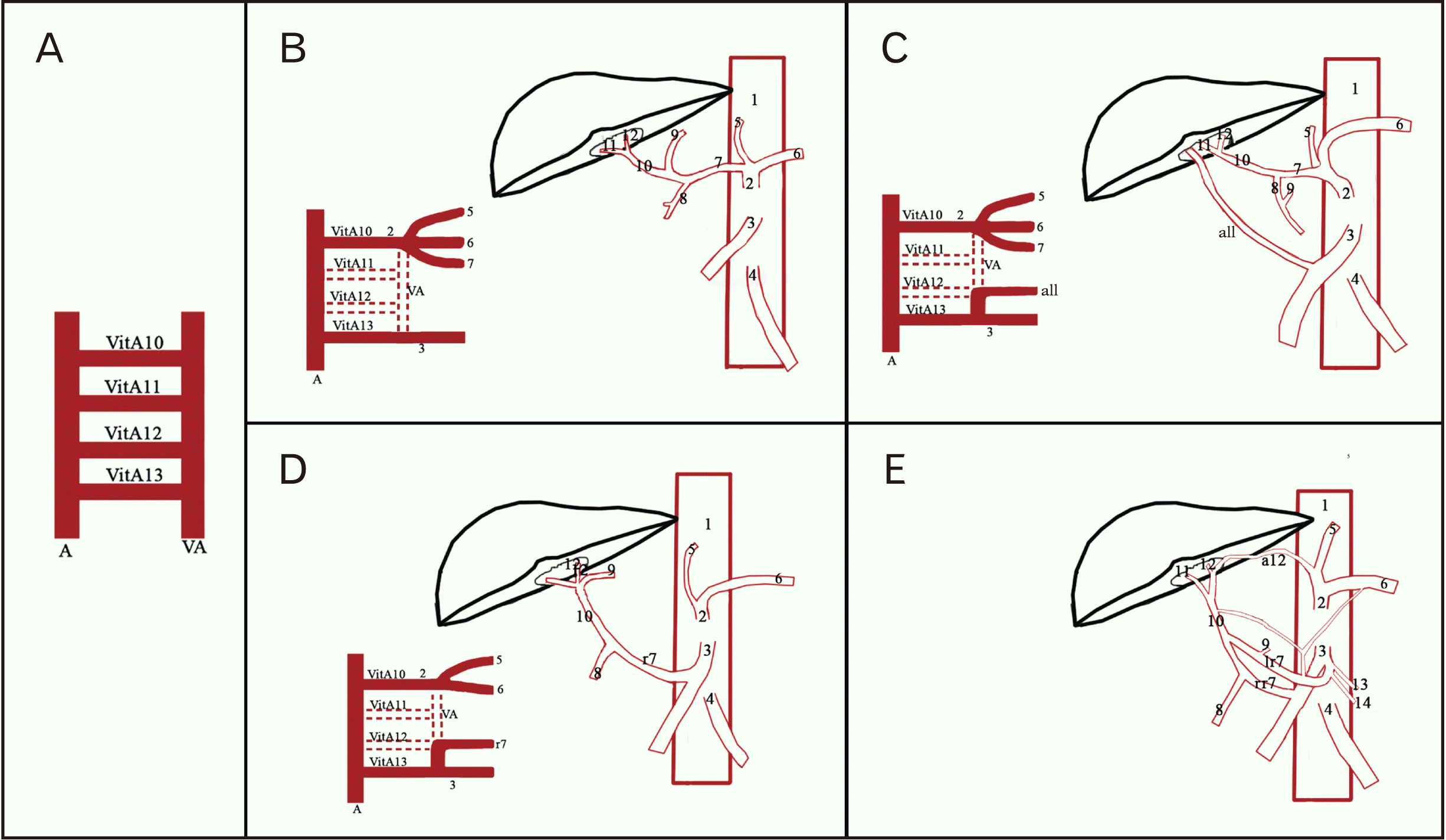
Table 1
Correlation of hepatic artery variant of the present study with Michels and Hiatt classification
| No. | Types of hepatic arterial anatomy | Michels classification (1966) [3] | Hiatt classification (1994) [4] | Present study (2022) |
|---|---|---|---|---|
| 1 | Normal anatomy | Type I (55.61%) | Type I (75.77%) | 91.43% |
| 2 | Replaced LHA branch LGA | Type II | Type II | - |
| 3 | Replaced RHA branch SMA | Type III | Type III | - |
| 4 | Replaced LHA & replaced RHA | Type IV | Type IV | - |
| 5 | Accessory LHA branch of LGA | Type V | Type II | - |
| 6 | Accessory RHA branch of SMA | Type VI (1.5%–7%) | Type III (10.6%) | 2.86% |
| 7 | Accessory LHA & accessory RHA | Type VII | Type IV | - |
| 8 | Accessory LHA & replaced RHA, Accessory RHA & replaced LHA | Type VIII | Type IV | - |
| 9 | CHA branch of SMA | Type IX (2.4%–5%) | Type V (1.5%) | 2.86% |
| 10 | RHA and LHA branch of LGA, CHA branch of LGA | Type X | - | - |
| 11 | CHA branch of aorta | - | Type VI | - |
| Not notified in Michels and Hiatt classification | ||||
| Replaced CHA branch of SMA anatomises with accessory LHA branch of LGA (not yet classified types) | 2.86% | |||




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download