Vaccine-induced thrombotic thrombocytopenia (VITT) is a rare adverse event following administration of adenovirus-based COVID-19 vaccines. It is potentially life-threatening as it may induce venous or arterial thrombosis leading to secondary hemorrhage [1]. The early reported cohorts on VITT from countries in the European Union demonstrated thrombosis at unusual sites being common, with cerebral venous sinus thrombosis (CVST) representing the vast majority of them [2-4].
According to a recent report, patients with VITT and CVST have a high mortality rate, and early detection and prompt initiation of treatment, significantly reduces mortality rates [5]. In this study, we report a case of VITT with fatal CVST complicated by secondary intracerebral hemorrhage, despite initial management.
A 33-year-old female with a medical history of bronchial asthma presented to the emergency department with persistent headaches and vomiting 10 days after receiving the booster dose (third dose) of COVID-19 vaccination [ChAdOx1 nCoV-19 (AstraZeneca) vaccine]. She had previously received 2 doses of the messenger RNA-based COVID-19 vaccine (Pfizer) without any complications. Before the visit to the emergency department, the patient was administered analgesics during the initial clinic visit, however, the symptoms persisted. The patient had no family history of thrombophilia or other identifiable risk factors for thrombosis. Physical examinations, including neurological examinations, were normal.
Her initial full blood count revealed isolated thrombocytopenia (12×109/L) with normal hemoglobin and white cell counts. Peripheral blood films revealed thrombocytopenia with large and giant platelets and no other significant findings. Computed tomography and venography (CTV) of the brain revealed extensive thrombosis of the cerebral venous sinuses involving the superior sagittal sinus, left transverse sinus, left sigmoid and straight sinus, and internal cerebral vein, with no intracerebral hemorrhage (Fig. 1). The D-dimer level was markedly elevated (18.7 mcg/mL) with a relatively preserved prothrombin time (PT) of 14 sec and an activated partial thromboplastin time (APTT) of 29 sec. Serum fibrinogen level was reduced to 0.7 g/L. The diagnosis of VITT was later confirmed by the detection of anti-platelet factor 4 (PF4) antibodies using enzyme-linked immunosorbent assay (ELISA).
The patient was treated with intravenous immunoglobulin (IVIG) at a dose of 1 g/kg body weight for 2 days, along with 500 mg of intravenous methylprednisolone daily for 3 days. A therapeutic dose of anticoagulation with fondaparinux was initiated, and intermittent cryoprecipitate infusions were administered to maintain fibrinogen level above 1.0 g/L. She responded well to the initial treatment, with resolution of her symptoms, and serial platelet count monitoring showed a sustained increase to a platelet count of 46×109/L after 2 doses of IVIG. Unfortunately, she had reduced consciousness level on the third day of treatment, and a repeated CT scan showed the presence of secondary intracerebral hemorrhage with a worsening midline shift on serial scans. Despite active management, she succumbed to her illness 2 days later.
VITT is an uncommon adverse event of adenovirus-based COVID-19 vaccines, first identified in March 2021, following widespread global vaccination efforts to counter the severe acute respiratory syndrome coronavirus 2 (SARS CoV 2) pandemic. It is estimated that the incidence of VITT is approximately 1:50,000, with a higher risk in younger age groups [5]. The incidence of VITT in various Asian population cohorts is generally lower than that in the Caucasian populations [6]. It is uncertain why the Asian population is generally less susceptible to developing VITT. This may be related to the lower incidence of venous thromboembolism (VTE) and heparin-induced thrombocytopenia (HIT) in Asians [7, 8]. In 2021, Malaysia reported three cases of VITT following the administration of 4.2 million doses of ChAdOx1 nCoV-19 (AstraZeneca) vaccine [9]. These patients with no history of CVST, received appropriate treatment and recovered well. A brief description of the confirmed cases is provided in Table 1. However, the true incidence may be under-reported, likely due to lack of public awareness and inadequate access to specialized laboratory testing.
In the early reported cohorts, thrombosis at unusual sites was predominant, with CVST being the most common site of thrombosis, although other sites, including deep vein thrombosis (DVT), pulmonary embolism (PE), and arterial thrombosis occurred either in isolation or concurrently [2-5]. CVST typically presents with severe persistent headaches with or without associated neurological deficits. It has a high mortality rate, especially in patients with hemorrhagic sequelae due to the backpressure of occluded vessels; hence, prompt initiation of treatment with steroids, IVIG, and anticoagulation is vital in its management. For severe cases that are refractory to the initial treatment, plasma exchange can be considered [10].
VITT shares many clinical and laboratory features with autoimmune heparin immune thrombocytopenia (HIT), including thrombosis with thrombocytopenia and the timing of its onset [11]. This has led to the pathophysiological description of a crosslink between proteins of adenovirus-based vaccines with PF4, forming multimolecular complexes, and the formation of anti-PF4 antibodies. The production of anti-PF4 antibodies may occur in vaccinated individuals, but does not necessarily result in thrombosis. In VITT, anti-PF4 antibodies bind to the multimolecular complex of the vaccine and PF4, ultimately leading to platelet activation. In addition, formation of neutrophil extracellular traps (NETs) promotes FcγIIa receptor activation. This results in profound activation of the coagulation system and consumption of coagulation factors, causing disseminated intravascular coagulation (DIC) [10-12].
Different case definitions have been proposed by different guidelines, many of which include laboratory features of thrombocytopenia, D-dimer elevation, and a positive ELISA test for anti-PF4 antibodies as criteria to stratify patients into definite, probable, possible, and unlikely VITT categories [1, 5]. In resource-limited countries such as Malaysia, accessibility to facilities with specialized laboratory testing, especially for anti-PF4 antibodies, may represent a diagnostic challenge. Despite this, treatment must be initiated based on clinical suspicion of possible and probable VITT without waiting for the confirmatory ELISA test, as patient outcomes may be severely compromised if intervention is delayed.
In conclusion, our case highlights that VITT is associated with high morbidity and mortality rates. With vaccinations being advocated as an important tools to address the COVID-19 pandemic, educational measures should be taken to promote public awareness and alert clinicians to have high clinical suspicion for early diagnosis. As, treatment with IVIG, anticoagulation, and steroids will significantly reduce morbidity and mortality. An established referral and management pipeline should be implemented in both primary and tertiary health care facilities.
Acknowledgments
The authors would like to thank Dr. Veena Selvaratnam, a consultant hematologist from Hospital Ampang, Malaysia, for providing clinical information on other confirmed cases in Malaysia and the medical team involved in the management of this patient. All authors contributed equally to the writing of this manuscript.
REFERENCES
1. Greinacher A, Langer F, Makris M, et al. 2022; Vaccine-induced immune thrombotic thrombocytopenia (VITT): update on diagnosis and management considering different resources. J Thromb Haemost. 20:149–56. DOI: 10.1111/jth.15572. PMID: 34693641. PMCID: PMC8646430.

2. Schultz NH, Sørvoll IH, Michelsen AE, et al. 2021; Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 384:2124–30. DOI: 10.1056/NEJMoa2104882. PMID: 33835768. PMCID: PMC8112568.

3. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. 2021; Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 384:2092–101. DOI: 10.1056/NEJMoa2104840. PMID: 33835769. PMCID: PMC8095372.
4. Scully M, Singh D, Lown R, et al. 2021; Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 384:2202–11. DOI: 10.1056/NEJMoa2105385. PMID: 33861525. PMCID: PMC8112532.
5. Pavord S, Scully M, Hunt BJ, et al. 2021; Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med. 385:1680–9. DOI: 10.1056/NEJMoa2109908. PMID: 34379914.

6. Boonyawat K, Angchaisuksiri P. 2022; Vaccine-induced immune thrombotic thrombocytopenia with ChAdOx1 nCoV-19 is rare in Asia. Res Pract Thromb Haemost. 6:e12644. DOI: 10.1002/rth2.12644. PMID: 35071968. PMCID: PMC8760608. PMID: b10dc7fe07da4a2eb6f4b3b0e8fdfcd5.

7. Lee LH, Gallus A, Jindal R, Wang C, Wu CC. 2017; Incidence of venous thromboembolism in Asian populations: a systematic review. Thromb Haemost. 117:2243–60. DOI: 10.1160/TH17-02-0134. PMID: 29212112.
8. Boonyawat K, Angchaisuksiri P, Aryurachai K, Chaiyaroj S, Ahmadi Z, Chong BH. 2014; Low prevalence of heparin-induced thrombocytopenia after cardiac surgery in Thai patients. Thromb Res. 134:957–62. DOI: 10.1016/j.thromres.2014.08.020. PMID: 25204999.

9. National Pharmaceutical Regulatory Agency, Ministry of Health, Malaysia. 2022. Summary report on adverse events following immunisation of COVID-19 vaccines in Malaysia. National Pharmaceutical Regulatory Agency (NPRA);Petaling Jaya, Malaysia: at https://www.npra.gov.my/index.php/en/. Accessed April 20, 2022.
10. Rizk JG, Gupta A, Sardar P, et al. 2021; Clinical characteristics and pharmacological management of COVID-19 vaccine-induced immune thrombotic thrombocytopenia with cerebral venous sinus thrombosis: a review. JAMA Cardiol. 6:1451–60. DOI: 10.1001/jamacardio.2021.3444. PMID: 34374713.

11. Klok FA, Pai M, Huisman MV, Makris M. 2022; Vaccine-induced immune thrombotic thrombocytopenia. Lancet Haematol. 9:e73–80. DOI: 10.1016/S2352-3026(21)00306-9. PMID: 34774202. PMCID: PMC8585488.
12. Arepally GM, Ortel TL. 2021; Vaccine-induced immune thrombotic thrombocytopenia: what we know and do not know. Blood. 138:293–8. DOI: 10.1182/blood.2021012152. PMID: 34323940. PMCID: PMC8172307.
Fig. 1
Contrast-enhanced CT brain and venography identified the presence of sagittal sinus (red arrow), torcula Herophili (green arrow), right and left transverse (orange and blue arrow) and left sigmoid sinus thrombosis.
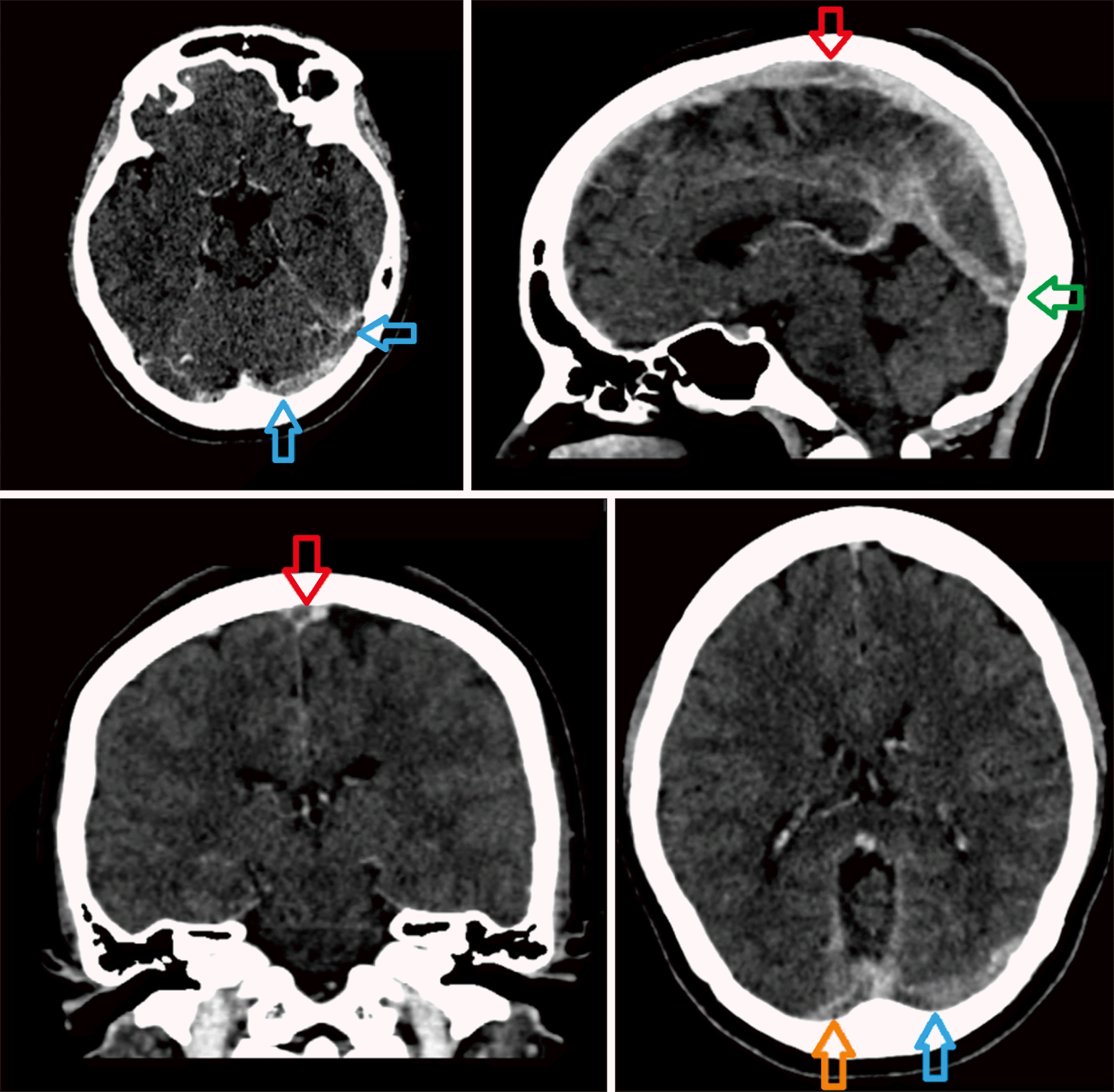
Table 1
Description of the clinical features of other confirmed VITT cases in Malaysia.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download