Myelodysplastic/myeloproliferative neoplasm with ring sideroblasts and thrombocytosis (MDS/ MPN-RS-T) is a unique, overlapping neoplasm. This neoplasm is characterized by features that include SF3B1-mutant MDS with ring sideroblasts and development of thrombocytosis secondary to the acquisition of signaling mutations, most commonly JAK2 V617F [1-3]. Here, we report a rare case of late-onset MDS/MPN-RS-T with triple driver mutations in SF3B1, CALR, and JAK2 V617F, at the age of 95. SF3B1 and CALR were co-dominant mutations, whereas JAK2 V617F was a low-level somatic mutation that was missed in next-generation sequencing (NGS) analysis.
A 95-year-old woman visited Soonchunhyang University Bucheon Hospital for the evaluation of dyspnea. She had suffered from anemia and heart failure over the past three months. A complete blood count revealed macrocytic anemia (hemoglobin, 6.1 g/dL) with a mean corpuscular volume as high as 99.4 fL, leukopenia (white blood cell, 3.7×109/L), and thrombocytosis (platelet count, 490×109/L). Serum biochemical analysis revealed normal folate and cobalamin levels. Haptoglobin and unconjugated bilirubin levels were also normal, and the direct Coombs test result was negative. There was no history of inflammatory or infectious disorders or any other causes of reactive thrombocytosis. Bone marrow analysis revealed dyserythropoiesis with multinuclear and megaloblastoid changes (Fig. 1A). Iron staining showed normal iron levels (grade 2), but ring sideroblasts in up to 20% of all erythroblasts (Fig. 1B). Blasts accounted for <5% of the total nucleated cells. A bone marrow aspirate smear showed the proliferation of large, atypical megakaryocytes (Fig. 1C). The bone marrow cellularity was normal, and reticulin and trichrome staining revealed no myelofibrosis.
The karyotype of the patient was normal. For thrombocytosis, an allele-specific, real-time polymerase chain reaction (PCR, BioSewoom, Seoul, Korea) for the JAK2 V617F mutation was performed using bone marrow aspirate, and the result was positive. The results from the NGS panel for MDS/MPN detected a Tier 1 SF3B1 R625H mutation and a Tier 1 CALR D373TfsTer57 mutation with variant allele frequencies of 36% and 32%, respectively (Fig. 1D, E). However, unlike the previous PCR result for the JAK2 V617F study, the mutation was negative in the targeted NGS panel test and in the repeat NGS test. The genomic data visualization tool Integrative Genomics Viewer (IGV) [4] identified a JAK2 V617F mutation with a low allele burden of less than 1% (Fig. 1F), lower than the variant calling cut-off (3%) used for NGS analysis in the laboratory. Based on these results, the patient was diagnosed with SF3B1/CALR/ JAK2 triple-mutated MDS/MPN-RS-T. The patient is currently receiving aspirin for thrombocytosis and a darbepoetin injection every 2 weeks for anemia. Although the requirement for red blood cells has decreased after the darbepoetin treatment, approximately 2 units of blood transfusion are still required per month.
Reporting of this case without informed consent was approved by the Institutional Review Board of Soonchunhyang University Bucheon Hospital (SCHBC 2022-02-023).
We present a rare case of MDS/MPN-RS-T with SF3B1, CALR, and JAK2 mutations in this study. This is an important finding given that the disease is defined by the specific presence of SF3B1 and JAK2 V617F mutations [2]. Other signaling mutations, such as MPL and CALR are infrequent (<5%) in MDS/MPN-RS-T [2]. Cases of MDS/MPN-RS-T with triple mutations have rarely been reported (Table 1) [5, 6]. Triple SF3B1/CARL/JAK2 mutations, as in the current patient, have previously only been described in one other case [5]. The affected individual had a triple mutation in the MDS/MPN-RS-T disease phase, but the JAK2 and CALR mutations weakened while the SF3B1 mutant clone strengthened as the patient developed myelofibrosis and acute myeloid leukemia [5]. Compared to the previous case [5], the current patient experienced late disease onset accompanied by leukopenia and mild thrombocytosis at the time of diagnosis, showing a tendency towards MDS features. To date, little is known about the prognosis of MDS/MPN- RS-T with triple mutations, making long-term follow-ups with our patient essential.
The JAK2 V617F allele burden in our case was very low (0.6%) compared to that of SF3B1 (36%) and CALR (32%) mutations. The antecedent relationship between these mutations was unclear at the time of the initial diagnosis. The low JAK2 mutant burden might be explained by pre-existing clonal hematopoiesis before overt signs of disease [7], followed by the acquisition of second oncogenic mutations. Meanwhile, in most patients with MDS/MPN-RS-T, it is believed that SF3B1-mutant MDS clonally evolves into MDS/MPN-RS-T with the acquisition of signaling mutations [1, 2, 5]. The JAK2 V617F mutation might have been acquired at the latest stage of the disease.
NGS plays an important role in the investigation of somatic mutations in hematological malignancies. Mutation frequency is a major problem in tumor mutation detection, and, as in the present case, detection of low-level (<1%) somatic mutations is a challenge for conventional NGS [8]. In addition to improving mutation calling performance by increasing sequencing depth, it is also important to verify the mutation using visualization tools, such as IGV, for important known driver mutations, regardless of the defined cut-off value for analysis.
In conclusion, we identified a rare case of MDS/MPN-RS-T with a triple SF3B1/CALR/JAK2 mutation. The presence of multiple mutations is rarely observed, and the molecular mechanisms causing molecular complexity and their consequent clinical impact is unclear. We believe that this report contributes to a better understanding of the clinical features and molecular basis of this rare type of MDS/ MPN-RS-T.
Acknowledgments
This study was supported by the Soonchunhyang University Research Fund (2022) and the National Research Foundation of Korea (NRF) grant, funded by the Ministry of Science and ICT (2021R1C1C1005725).
REFERENCES
1. Patnaik MM, Tefferi A. 2021; Myelodysplastic syndromes with ring sideroblasts (MDS-RS) and MDS/myeloproliferative neoplasm with RS and thrombocytosis (MDS/MPN-RS-T) - "2021 update on diagnosis, risk-stratification, and management". Am J Hematol. 96:379–94. DOI: 10.1002/ajh.26090. PMID: 33428785.
2. Patnaik MM, Lasho TL. 2020; Genomics of myelodysplastic syndrome/myeloproliferative neoplasm overlap syndromes. Hematology Am Soc Hematol Educ Program. 2020:450–9. DOI: 10.1182/hematology.2020000130. PMID: 33275756. PMCID: PMC7727543.
3. Swerdlow SH, Campo E, Harris NL, et al. 2017. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th ed. IARC Press;Lyon, France: p. 93–4.
4. Thorvaldsdóttir H, Robinson JT, Mesirov JP. 2013; Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform. 14:178–92. DOI: 10.1093/bib/bbs017. PMID: 22517427. PMCID: PMC3603213.

5. Yasuda H, Morishita S, Mori Y, et al. 2021; JAK2/CALR/SF3B1 triple-mutated myelodysplastic/myeloproliferative neoplasm with ring sideroblasts and thrombocytosis evolving to myelofibrosis and SF3B1 single-mutated acute myeloid leukemia: evidence of a pre-JAK2 clone. Leuk Res. 100:106496. DOI: 10.1016/j.leukres.2020.106496. PMID: 33373831.

6. Ye MT, Wang Y, Wang SA, et al. 2020; Concurrent JAK2 V617F and MPL W515L in myelodysplastic/myeloproliferative neoplasm with ring sideroblasts and thrombocytosis. Leuk Lymphoma. 61:963–6. DOI: 10.1080/10428194.2019.1691202. PMID: 31750750.
7. Nielsen C, Bojesen SE, Nordestgaard BG, Kofoed KF, Birgens HS. 2014; JAK2V617F somatic mutation in the general population: myeloproliferative neoplasm development and progression rate. Haematologica. 99:1448–55. DOI: 10.3324/haematol.2014.107631. PMID: 24907356. PMCID: PMC4562533.

8. Stead LF, Sutton KM, Taylor GR, Quirke P, Rabbitts P. 2013; Accurately identifying low-allelic fraction variants in single samples with next-generation sequencing: applications in tumor subclone resolution. Hum Mutat. 34:1432–8. DOI: 10.1002/humu.22365. PMID: 23766071.

Fig. 1
Hematological and molecular characteristics of the present MDS/MPN-RS-T patient with triple SF3B1, CALR, and JAK2 driver mutations. (A) Wright–Giemsa stain (×400) of bone marrow aspirate showing dyserythropoiesis with multinuclearity and megaloblastoid changes (arrows). (B) Iron stain (×1,000) showing ring sideroblasts (arrows). (C) Wright–Giemsa stain (×200) showing increased cellularity and proliferation of large pleomorphic megakaryocytes. (D–F) Integrative Genomics Viewer snapshot of SF3B1 missense mutation (NC_000002.11:g.198267483C>T, R625H) (D), CALR frameshift mutation (NC_000019.9:g.13054590del, D373TfsTer57) (E), and JAK2 missense mutation (NC_000009.11: g.5073770G>T, V617F) (F) identified by next-generation sequencing (arrowheads).
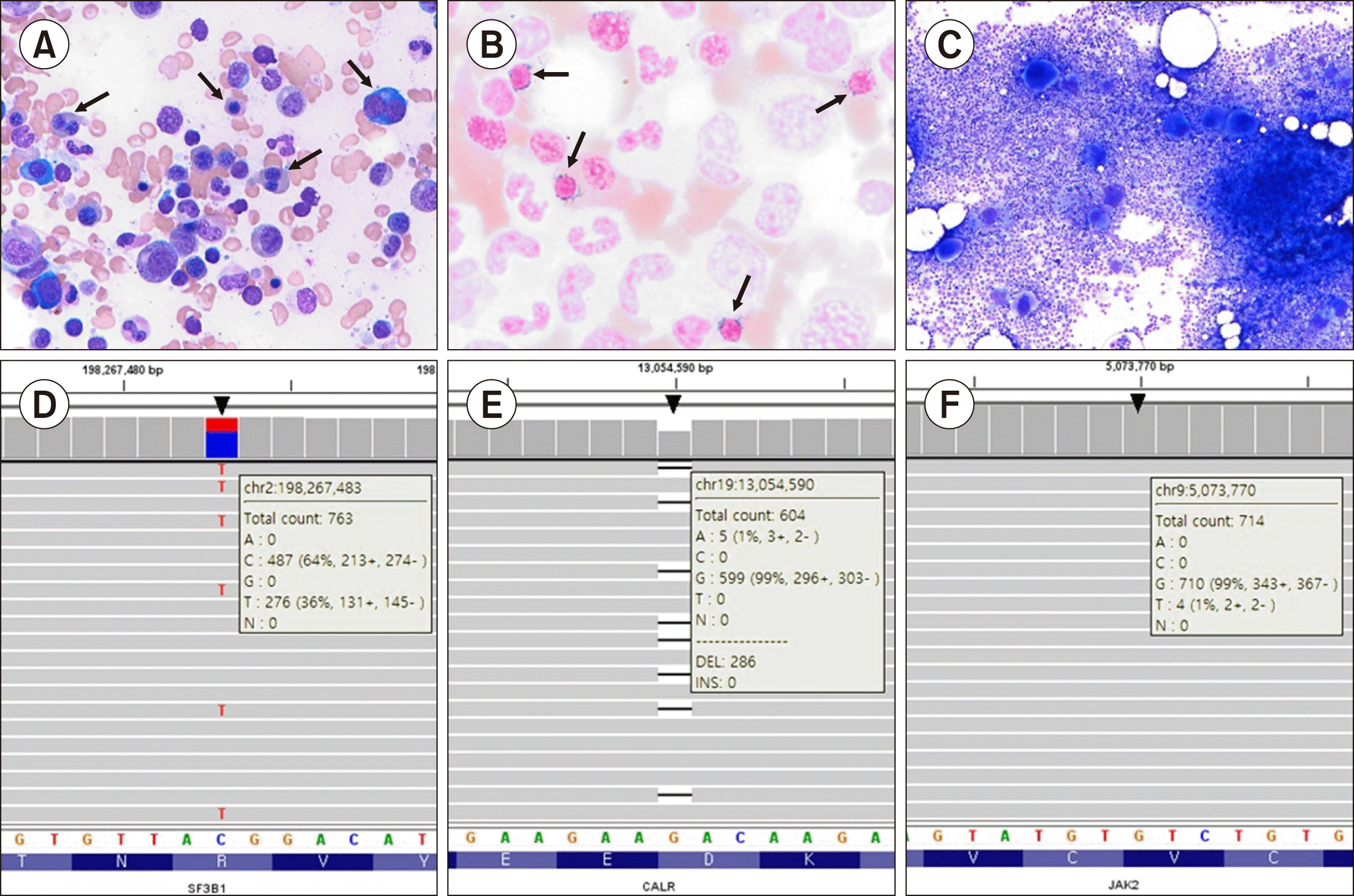
Table 1
Summary of patients with MDS/MPN-RS-T carrying triple driver mutations.
| This study | Yasuda et al. [5] | Ye et al. [6] | |
|---|---|---|---|
| Age at diagnosis (yr) | 95 | 60 | 68 |
| Sex | Female | Male | Female |
| Splenomegaly | No | ND | No |
| Complete blood count | |||
| Hb (g/dL) | 6.1 | 9.4 | 9.0 |
| WBC (×109/L) | 3.7 | 6.5 | 4.4 |
| Platelets (×109/L) | 490 | 775 | 658 |
| Bone marrow features | |||
| Ring sideroblasts (%) | 20 | >15 | 70 |
| Megakaryocytic hyperplasia | Yes | Yes | Yes |
| Fibrosis | No | ND | Yes |
| Cytogenetics | Normal | Normal | Normal |
| Mutation (allele burden) | |||
| SF3B1 mutation | R625H (36%) | R625C (40%) | K700E (36%) |
| MPN-associated driver mutation | |||
| JAK2 | V617F (1%) | V617F (5–10%) | V617F (20%) |
| CALR | D373TfsTer57 (32%) | E378RfsTer45 (5–10%) | Wild type |
| MPL | Wild type | Wild type | W515L (4%) |
| Treatment | Aspirin | Hydroxyurea, anagrelide | Darbepoetin alpha, hydroxyurea, and aspirin |
| Transfusion dependency | Yes | ND | ND |
| Follow-up/outcome | Alive |
Dead Myelofibrosis/AML progression |
ND |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download