Abstract
Background
Data on the association between coronavirus disease 2019 (COVID-19) and the epidemiology and outcomes of hematological malignancies are limited. Hence, the present study aimed to assess the imaging findings using chest multidetector computed tomography (MDCT) in patients with hematologic malignancies who developed COVID-19 pneumonia.
Methods
This retrospective study included two groups, the first group consisted of COVID-19 infected patients with hematologic malignancies (100 patients), while the second group consisted of COVID-19 infected patients without hematologic malignancies or other comorbidities (100 patients). The hematological malignancies included in this study were non-Hodgkin’s lymphoma (40 patients), acute myeloid leukemia (25 patients), chronic lymphocytic leukemia (15 patients), multiple myeloma (10 patients), Hodgkin’s lymphoma (8 patients), and myelodysplastic syndrome (2 patients). Chest multidetector CT imaging was performed in all patients to assess for ground-glass opacity, consolidation, pleural effusion, and airway abnormalities.
Results
More than one CT finding was reported in each patient. No significant difference was observed in the ground-glass opacities (P=0.0594), nodule formation (P=0.2278), or airway thickening/dilatation (P=0.0566) between the two groups; meanwhile, a significant difference was observed in the degree of consolidation, the number of lobes affected, and pleural effusion (P=0.0001) as well as in the total lung severity (P=0.0001); minimal, mild, and severe affection rates; and (P=0.0047) moderate affection rates.
Conclusion
Early and reliable diagnosis of lung disease in COVID-19-infected patients may be achieved through multidetector CT imaging. Patients with hematological malignancies are more likely to have severe COVID-19 pneumonia, and radiologists should recognize the CT characteristics of this infection.
The first case of pneumonia attributed to coronavirus disease-2019 (COVID-19) was reported in Wuhan, Hubei, China, in December 2019 [1]; in January 2020, the World Health Organization (WHO) declared the COVID-19 outbreak as a global health emergency [2]. The presenting symptoms of COVID-19 typically include fever, dry cough, and dyspnea; the other non-specific manifestations include headache, fatigue, and muscle soreness. More severe forms of infection can result in severe pneumonia, acute respiratory distress syndrome, and even death [3].
COVID-19 is diagnosed using real-time polymerase chain reaction (RT-PCR) test, despite its relatively low detection rates and lower sensitivity compared with chest computed tomography (CT). Therefore, chest CT is currently used to screen for COVID-19 in clinically suspicious individuals with false-negative RT-PCR results. In addition to its diagnostic role in COVID-19, CT imaging is used in patient follow-up to evaluate the response to therapy [4-6]. This study only included hematologic malignant patients with confirmed coronavirus disease 2019 (COVID-19) pneumonia, in order to examine and evaluate their chest CT findings.
This retrospective study was approved by the Institutional Research Ethics Review Committee; the requirement for obtaining informed consent was waived due to the retrospective nature of this study. One hundred patients with an average age of 44.67 years (range, 25–75 yr) with hematologic malignancies were diagnosed with COVID-19 by RT-PCR (rRT_q PCR by genesigⓇ real-time PCR, Primerdesign Ltd, UK); the patients’ baseline demographic findings are described in Table 1. The majority of patients presented with fever, cough, dyspnea, and myalgia (Table 2). Forty individuals were subjected to microbiological testing for the diagnosis of bacterial and fungal infections. Patients with pneumonia caused by common bacterial or viral infections were excluded from the study. The duration of this study was 3 months. These patients had the following hematological malignancies: non-Hodgkin’s lymphoma (40 patients), acute myeloid leukemia (25 patients), chronic lymphocytic leukemia (15 patients), multiple myeloma (10 patients), Hodgkin’s lymphoma (8 patients), and myelodysplastic syndrome (2 patients). Thirty patients had a history of comorbidities. Seven patients had a combination of cardiomyopathy, diabetes mellitus, and hypertension, six patients had diabetes, and another six patients had hypertension. Five patients had cardiomyopathy and hypertension. Five patients were clinically obese, and one patient was alcoholic. Systemic hypertension was observed in these patients. A control group of 100 patients (median age, 46.2 yr) with confirmed COVID-19 diagnosed by RT-PCR but no hematologic malignancies or other comorbidities was selected to match the study group.
Multidetector CT (MDCT) chest examination was performed using four scanners: Brilliance 64 (Philips Healthcare), SOMATOM go.Now (Siemens Healthcare), Symbia Intevo (Siemens Healthcare), and 16-slice Optima (GE Healthcare). Initial non-enhanced chest CT scans were performed within 10 days (mean, 6 days) after disease onset. End-inspiratory images were taken using standard CT with the patient in the supine position at 120 kVp, automatic mA adjustment, 3-mm slice thickness, 1-mm section reconstruction, 0.75–1.5 pitch, and 0.625 mm collimation. The images were examined on mediastinal and lung windows (window widths: 350 HU and 1,600 HU; levels: 400 HU and –600 HU, respectively).
Following the diagnosis of COVID-19, the initial MDCT images were reviewed to assess for ground-glass opacity (GGO), consolidation, the number of lung lobes affected, interlobular septal thickening, nodule formation, pleural effusion, and airway abnormalities such as airway wall thickening and dilatation, in addition to the presence of any accompanying hilar, mediastinal, pleural, or pericardial findings. Patients showing GGOs and consolidation were assessed for laterality of lung disease, with the outer one-third of the lung being described as peripheral and the remainder as central. Bernheim et al. [7] used a severity scale to assess the severity of lung disease; the severity of affection in each of the five lung lobes was assessed and classified as no affection (0% involvement, score 0), minimal (1–25% involvement, score 1), mild (26–50% involvement, score 2), moderate (51–75% involvement, score 3), or severe (76–100% involvement, score 4). The overall lung “total severity score” was calculated as the sum of the five lobe ratings within a range of 0 to 20. A score of 1–5 was considered minimum, a score of 6–10 was considered mild, a score of 11–15 was considered moderate, and a score of 16–20 was considered severe. Lymphadenopathy-related COVID-19 has been defined as the appearance of a newly developed inflammatory lymph node with a short-axis diameter greater than 10 mm during infection. Statistical analysis was performed using the McNemar’s chi-square test, and a P-value of <0.05 was considered significant.
The study included 100 participants with hematologic malignancies (62 men and 38 women) and 100 individuals with no malignancies or comorbidities, which were selected to match the study group. In each patient, more than one CT finding was observed. GGO was detected in 100 out of 100 and 95 out of 100 control groups (P=0.0594). Furthermore, consolidation was reported in 80 patients from the study group and in 27 patients from the control group (P=0.0001), while airway wall thickening and dilatation were detected in 70 patients from the study group and 56 patients from the control group (P=0.0566). Nodules were found in 18 of the 100 patients from the study group and in 11 of the 100 patients from the control group (P=0.2278). Pleural effusion was observed in the 50 of the 100 patients from the study group and 7 of the 100 patients from the control group (P=0.0001); meanwhile, interlobular septal thickening was identified in the 32 of the 100 and 24 of the 100 patients from the control groups (P=0.2702). Lymphadenopathy was noted in 16 patients from the study group and in 5 patients from the control group (P=0.0192) (Fig. 1–4, Table 3).
Central and peripheral lung affection was detected in 60 patients from the study group and 37 patients from the control group (P=0.0018). Moreover, 30 of the 100 patients from the study group and 58 of the 100 patients from the control group showed peripheral affection (P=0.0001), while the 10 of the 100 patients from the research group and 5 of the 100 patients from the control group demonstrated central affection (P=0.2828). Of the 500 lung lobes, 450 were affected in the research group and 395 in the control group, respectively (P=0.0001). The incidence rates of lobar involvement were described in Table 3, 4. Meanwhile, 45 patients from the study group and 15 patients from the control group had severe lung affection (P=0.0001), 45 from the study group and 25 from the control group had moderate lung affection (P=0.0047), 7 from the research group and 30 from the control group had mild lung affection (P=0.0001), and 2 from the research group and 30 from the control group had minimal lung affection (P=0.0001). Pericardial effusion was noted in 10 patients from the study group and none from the control group. A difference was not found in the CT images according to sex, age group, and comorbidity in COVID-19 patients, and no difference was also found in the CT images of each hematologic malignancy. Death due to the detrimental effects of COVID-19 occurred in of 22 of 100 patients, all of whom had comorbidities, including cardiomyopathy, diabetes mellitus, hypertension (7 patients), diabetes mellitus (4 patients), hypertension (4 patients), cardiomyopathy plus hypertension (4 patients), obesity (2 patients), and alcoholism (1 patient).
The early detection of COVID-19 is essential for providing prompt treatment, particularly in patients with COVID-19 pneumonia. This study examined 100 patients with hematologic malignancy and confirmed COVID-19 pneumonia, most of whom (62/100) were men. This finding is consistent with that of a previous study [8], which reported a male affection rate of 62.9%. Understanding the clinical and chest CT findings of COVID-19 is crucial for the early detection of infection and assessment of disease response [9, 10].
For the assessment of COVID-19, chest CT has a higher sensitivity compared with RT-PCR assay [6]. COVID-19 pneumonia was identified in all 100 patients in the current investigation, based on typical CT findings. The increased accuracy of CT may provide a basis for its standard use in the diagnosis of COVID-19. These findings are consistent with those of previous studies [10-12].
The most prevalent chest CT findings are bilateral multifocal GGOs with patchy consolidations and pronounced peripheral subpleural distribution [8, 11, 13]. Accounts from the current study coincided with these results in terms of GGOs (100/100 patients) and consolidation (80/100 patients). As regards the laterality of involvement, the lesions showed both central and peripheral distribution in 60 of the 100 patients, peripheral distribution in 30 of the 100 patients, and central distribution in 10 of the 100 patients. This disparity may be attributed to the geographical distribution of the selected group of patients with hematologic malignancies.
COVID-19-associated thoracic lymphadenopathy was detected in 16 of 100 patients; however, no lung cavitation or calcification was detected in the patient group. These results coincide with reports of the control group and of previous studies [12, 14-18]. However, pleural effusion was detected in 50 of the 100 study patients, which was in conflict with that observed in the control group and previous studies [19, 20], a difference possibly attributable to the side effects of chemotherapy rather than the COVID-19 itself. Zhou et al. [8] (2020) found pleural effusion in 9.7% of the patients, while another study reported an interlobular septal thickening in 70.6% and pulmonary nodules in 21.5% of the patients [11]. By contrast, the current study reported an interlobular septal thickening in 32 of the 100 patients and nodule formation in 18 of the 100 patients.
COVID-19 is typically diagnosed through review of epidemiological history, assessment of clinical symptoms, evaluation of imaging findings, and RT-PCR testing. The chest results of COVID-19 patients often mimic those of patients with other viral diseases, including the influenza A (H1N1) virus infection, common cold, and other coronavirus illnesses such as severe acute respiratory distress syndrome and Middle East respiratory syndrome [20-26]. As a result, a detailed history of the close interaction with a verified or suspected patient is critical for the diagnosis, and an RT-PCR test should be performed in individuals with characteristic clinical and radiological manifestations indicative of COVID-19 for verification.
Cancer patients are more likely to acquire COVID-19- related severe illness [27]. The present study corroborates these findings and reported 45 patients showing severe disease and another 45 showing moderate disease. The severity of COVID-19 pneumonia was more pronounced in the research group than in the control group, most likely because of the low immunity of patients with malignancy. Although the frequency of untimely death in individuals with COVID-19-infected cancer in China was reported to be 28.6% [27], findings from the present study show relatively similar results, with death occurring in 22 of the 100 patients.
The shortage of long-term follow-up and the retrospective nature of the analysis are the limitations of the present study.
Multidetector CT scan was used to establish an early and reliable diagnosis of lung disease caused by COVID-19. A significant pattern of COVID-19 pneumonia has been detected in individuals with hematologic cancer. The typical CT characteristic of COVID-19 pneumonia is presence of GGOs. Radiologists should be aware of the CT findings of this type of viral infection in patients with hematologic malignancies, with prompt consideration of the diagnosis of COVID-19 pneumonia.
ACKNOWLEDGMENTS
We thank the Research Support Office, Faculty of Medicine, Mansoura University, Egypt, for their assistance in editing this manuscript.
REFERENCES
1. Zhu N, Zhang D, Wang W, et al. 2020; A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 382:727–33. DOI: 10.1056/NEJMoa2001017. PMID: 31978945. PMCID: PMC7092803.

2. Mahase E. 2020; China coronavirus: WHO declares international emergency as death toll exceeds 200. BMJ. 368:m408. DOI: 10.1136/bmj.m408. PMID: 32005727.

3. Wang W, Tang J, Wei F. 2020; Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J Med Virol. 92:441–7. DOI: 10.1002/jmv.25689. PMID: 31994742. PMCID: PMC7167192.

4. Fang Y, Zhang H, Xie J, et al. 2020; Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 296:E115–7. DOI: 10.1148/radiol.2020200432. PMID: 32073353. PMCID: PMC7233365.
5. Wang Y, Hou H, Wang W, Wang W. 2020; Combination of CT and RT-PCR in the screening or diagnosis of COVID-19. J Glob Health. 10:010347. DOI: 10.7189/jogh.10.010347. PMID: 32373325. PMCID: PMC7183249.

6. Ai T, Yang Z, Hou H, et al. 2020; Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 296:E32–40. DOI: 10.1148/radiol.2020200642. PMID: 32101510. PMCID: PMC7233399.
7. Bernheim A, Mei X, Huang M, et al. 2020; Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 295:200463. DOI: 10.1148/radiol.2020200463. PMID: 32077789. PMCID: PMC7233369.

8. Zhou S, Wang Y, Zhu T, Xia L. 2020; CT features of coronavirus disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. AJR Am J Roentgenol. 214:1287–94. DOI: 10.2214/AJR.20.22975. PMID: 32134681.

9. Koo HJ, Lim S, Choe J, Choi SH, Sung H, Do KH. 2018; Radiographic and CT features of viral pneumonia. Radiographics. 38:719–39. DOI: 10.1148/rg.2018170048. PMID: 29757717. PMCID: PMC7336757.

10. Zu ZY, Jiang MD, Xu PP, et al. 2020; Coronavirus disease 2019 (COVID-19): a perspective from China. Radiology. 296:E15–25. DOI: 10.1148/radiol.2020200490. PMID: 32083985. PMCID: PMC7233368.

11. Li Y, Xia L. 2020; Coronavirus disease 2019 (COVID-19): role of chest CT in diagnosis and management. AJR Am J Roentgenol. 214:1280–6. DOI: 10.2214/AJR.20.22954. PMID: 32130038.

12. Cheng Z, Lu Y, Cao Q, et al. 2020; Clinical features and chest CT manifestations of coronavirus disease 2019 (COVID-19) in a single-center study in Shanghai, China. AJR Am J Roentgenol. 215:121–6. DOI: 10.2214/AJR.20.22959. PMID: 32174128.

13. Kanne JP. 2020; Chest CT findings in 2019 novel coronavirus (2019- nCoV) infections from Wuhan, China: key points for the radiologist. Radiology. 295:16–7. DOI: 10.1148/radiol.2020200241. PMID: 32017662. PMCID: PMC7233362.

14. Pan F, Ye T, Sun P, et al. 2020; Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19). Radiology. 295:715–21. DOI: 10.1148/radiol.2020200370. PMID: 32053470. PMCID: PMC7233367.

15. Chung M, Bernheim A, Mei X, et al. 2020; CT imaging features of 2019 novel coronavirus (2019-nCoV). Radiology. 295:202–7. DOI: 10.1148/radiol.2020200230. PMID: 32017661. PMCID: PMC7194022.

16. Song F, Shi N, Shan F, et al. 2020; Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 295:210–7. DOI: 10.1148/radiol.2020200274. PMID: 32027573. PMCID: PMC7233366.

17. Pan Y, Guan H, Zhou S, et al. 2020; Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol. 30:3306–9. DOI: 10.1007/s00330-020-06731-x. PMID: 32055945. PMCID: PMC7087663.

18. Liu T, Huang P, Liu H, et al. 2020; Spectrum of chest CT findings in a familial cluster of COVID-19 infection. Radiol Cardiothorac Imaging. 2:e200025. DOI: 10.1148/ryct.2020200025. PMID: 33778542. PMCID: PMC7233390.
19. Kong W, Agarwal PP. 2020; Chest imaging appearance of COVID-19 infection. Radiol Cardiothorac Imaging. 2:e200028. DOI: 10.1148/ryct.2020200028. PMID: 33778544. PMCID: PMC7233424.

20. Li X, Zeng X, Liu B, Yu Y. 2020; COVID-19 infection presenting with CT halo sign. Radiol Cardiothorac Imaging. 2:e200026. DOI: 10.1148/ryct.2020200026. PMID: 33778543. PMCID: PMC7194018.

21. Zu ZY, Jiang MD, Xu PP. 2020; Radiological diagnosis of new coronavirus infected pneumonitis: expert recommendation from the Chinese Society of Radiology. Chin J Radiol. 54:E001.
22. El-Badrawy A, Zeidan A, Ebrahim MA. 2012; 64 multidetector CT findings of influenza A (H1N1) virus in patients with hematologic malignancies. Acta Radiol. 53:662–7. DOI: 10.1258/ar.2012.120038. PMID: 22734081.

23. Malainou C, Herold S. 2019; Influenza. Internist (Berl). 60:1127–35. DOI: 10.1007/s00108-019-00670-6. PMID: 31478058.

24. de Wit E, van Doremalen N, Falzarano D, Munster VJ. 2016; SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 14:523–34. DOI: 10.1038/nrmicro.2016.81. PMID: 27344959. PMCID: PMC7097822.

25. Hui DSC, Zumla A. 2019; Severe acute respiratory syndrome: historical, epidemiologic, and clinical features. Infect Dis Clin North Am. 33:869–89. DOI: 10.1016/j.idc.2019.07.001. PMID: 31668196. PMCID: PMC7127569.
26. Chan JF, Lau SK, To KK, Cheng VC, Woo PC, Yuen KY. 2015; Middle East respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clin Microbiol Rev. 28:465–522. DOI: 10.1128/CMR.00102-14. PMID: 25810418. PMCID: PMC4402954.

27. Gosain R, Abdou Y, Singh A, Rana N, Puzanov I, Ernstoff MS. 2020; COVID-19 and cancer: a comprehensive review. Curr Oncol Rep. 22:53. DOI: 10.1007/s11912-020-00934-7. PMID: 32385672. PMCID: PMC7206576.

Fig. 1
63-year-old male patient with coronavirus disease (COVID-19) and history of chronic lymphocytic leukemia. Multidetector CT scan of the chest (A) showed bilateral pleural effusions (asterisks) and malignant lymphadenopathy in the superior mediastinum (arrows); (B) bilateral ground-glass opacities (asterisks); (C) bronchial dilatation (arrow), bilateral ground-glass opa-cities (asterisks), and right pneum-onic consolidation (right triangles); and (D) bilateral ground-glass opa-cities (asterisks), bronchial dilatation (arrows), and interlobular septal thickening.
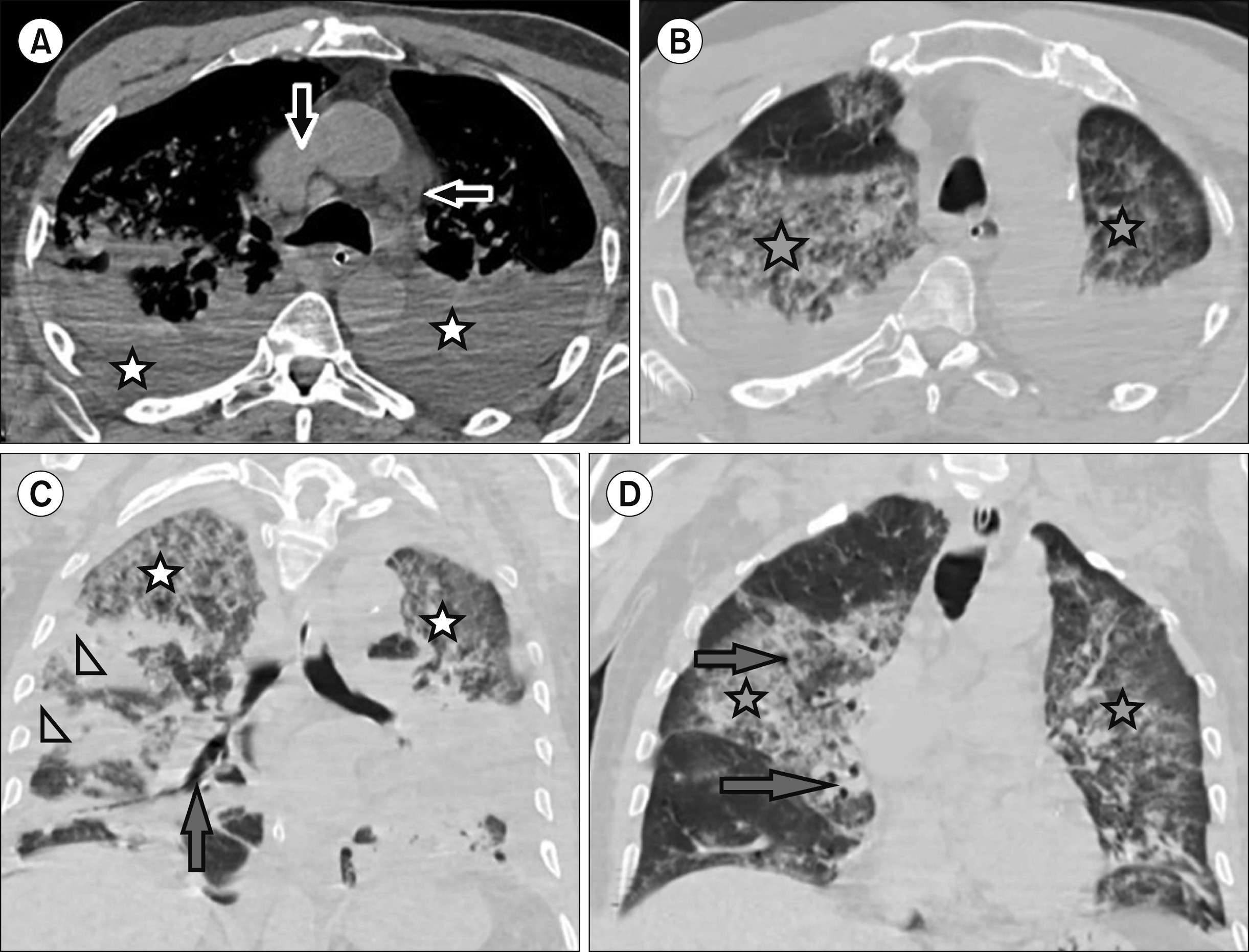
Fig. 2
36-year-old woman with coronavirus disease (COVID-19) and history of non-Hodgkin lym-phoma. Multidetector CT scan of the chest (A, B) showed multiple bilateral axillary and superior med-iastinal looking malignant lymphad-enopathy (arrows) and (C, D) ground-glass opacities (asterisks), interlobular septal thick-ening, and bronchial dilatation (arrow). Follow- up MDCT scan of the chest after 11 days (E, F) showed improvement in ground-glass opacities (asterisks).

Fig. 3
55-year-old man with coronavirus disease (COVID-19) and history of acute myeloid leukemia. Multidetector CT scan of the chest (A, B) showed ground- glass opacities (arrows) and nodules (triangles). Follow-up MDCT scan of the chest after 10 days (C, D) showed increased ground-glass opacities (arrows) with newly developed mild left pleural effusion (asterisk).
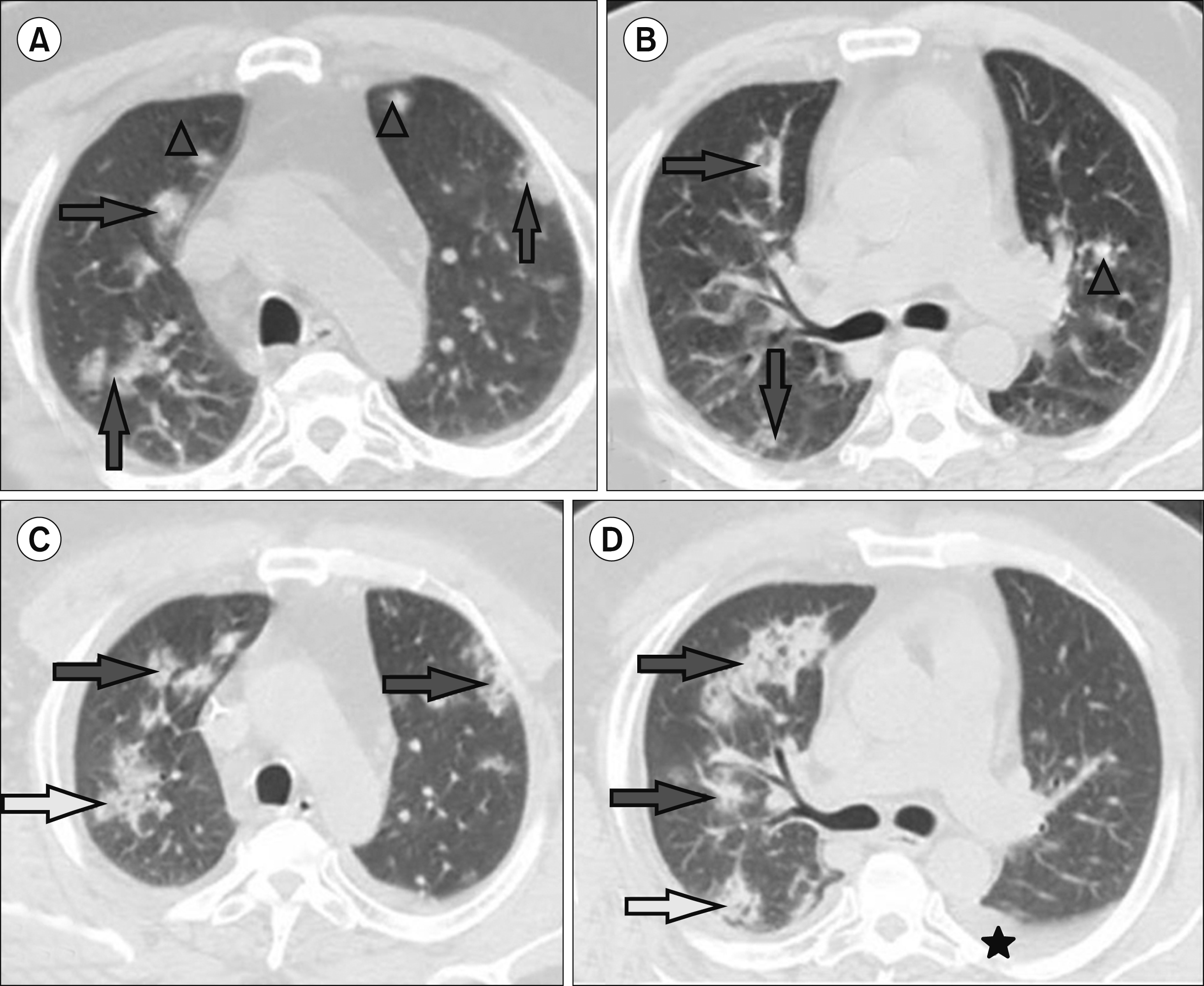
Fig. 4
45-year-old woman with coronavirus disease (COVID-19) and history of multiple myeloma. Multidetector CT scan of the chest (A) showed dilated cardiac chamber, pericardial (arrow), and pleural effusions (asterisks) and (B, C) bronchial dilatation (triangle) and ground-glass opacities (arrows). Follow-up MDCT scan of the chest after 8 days (D) showed progression of pericardial effusion (arrow) and pleural effusion (asterisks) and (E, F) Ground-glass opacities (arrows) and a newly developed consolidation (oval).

Table 1
Baseline demographic findings of 100 patients with hematologic malignancies.
Table 2
Clinical presentation of COVID-19 pneumonia in 100 patients with hematologic malignancies and 100 patients from the control group.
Table 3
Chest MDCT findings of COVID-19 pneumonia in 100 patients with hematologic malignancies and 100 patients from the control group.
Table 4
Incidence of lobar involvement of COVID-19 pneumonia in 100 patients with hematologic malignancies and 100 patients from the control group.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download