Dear Editor,
Hemophilia A is an X-linked bleeding disorder caused by mutations in factor VIII (FVIII) genes. The standard treatment is prophylactic administration of FVIII concentrate. However, a complication of FVIII replacement is the development of antibody against the replacement FVIII, which may inhibit the factor. One promising novel therapy is emicizumab (Hemlibra; F. Hoffmann–La Roche Ltd., Basel, Switzerland) [1]. Emicizumab exhibits a strong procoagulant effect on activated partial thromboplastin time (aPTT); a substantial shortening in aPTT is observed at low circulating emicizumab levels [2]. Guidelines for monitoring during treatment recommend that single-factor assays should use chromogenic substrate assays (CSAs) not affected by emicizumab [3-5]. We evaluated the analytical performance of a chromogenic FVIII activity assay employing bovine reagents. We assessed the correlation between the CSA and a one-stage clotting assay (OSA) for measuring FVIII activity and FVIII-inhibitor levels in patients with hemophilia treated with or without emicizumab.
The study included 109 plasma samples collected from 52 patients prescribed monitoring of hemophilia A that exhibited FVIII activity or FVIII inhibitors between April 2020 and August 2021 in Kyung Hee University Hospital at Gangdong, Seoul, Korea. The results of FVIII activity and FVIII inhibitors in patients treated with emicizumab were analyzed separately. The study was approved by the Ethics Committee of Gangdong Kyung Hee University Hospital (KHNMC 2022-01-028). The requirement for informed consent was waived.
The CSA was performed using a CN-6000 analyzer (Sysmex Corp., Kobe, Japan) with the Factor VIII Chromogenic Assay (Siemens Healthcare Diagnostics, Marburg, Germany). The OSA was performed using an ACL TOP 500 analyzer (Werfen, Milan, Italy) with HemosIL Synthasil and Factor VIII Deficient Plasma (Instrumentation Laboratory, Bedford, MA, USA) and the CN-6000 analyzer using actin FSL and coagulation FVIII deficient plasma (Siemens Healthcare Diagnostics). Statistical analyses were performed using SPSS v18.0 (SPSS Inc., Chicago, IL, USA) and MedCalc v20.015 (MedCalc Software, Ostend, Belgium).
The within-run coefficient of variance percent of CSA on the CN-6000 was 2.7% for the normal controls and 1.6% for the pathologic controls. Minimal carryover results were obtained (0.79%). The CSA demonstrated good linearity (R2=0.9975). The mean values of FVIII activity (N=79) from patients not receiving non-factor treatment were 17.29±33.13% for ACL TOP OSA, 14.72±31.17% for CN-6000 OSA, and 11.18±25.07% for CN-6000 CSA. Strong correlations were observed between ACL TOP OSA and CN-6000 OSA, ACL TOP OSA and CN-6000 CSA, and CN-6000 OSA and CN-6000 CSA (Fig. 1A, C, and E, respectively). The agreement was high according to the Bland–Altman plots (Fig. 1B, D, and F). All samples showing values exceeding the 95% CI of the limits of agreement (LOA) on the Bland–Altman plots were from patients receiving B-domain-deleted third-generation recombinant FVIII (GreenGene F and Xyntha).
The median levels of FVIII inhibitors (N=26) from patients not receiving non-factor treatment were 8.0 Bethesda units (BU) for ACL TOP OSA and 11.27 BU for CN-6000 CSA. A significant correlation existed between ACL TOP OSA and CN-6000 CSA (rho=0.8940, P=0.01). Bland–Altman analyses showed ACL TOP OSA and CN-6000 CSA had a mean difference of −11.6 BU, and LOA was −99.22 to 75.97 BU. The LOA demonstrated a narrow value at <200 BU of the FVIII inhibitor, indicating a good agreement between CSA and OSA. All CSA values were <1.5% for patients receiving emicizumab (N=4), but OSA values ranged 196.4%–617.9%, showing contrasting results (Table 1). The CSA results revealed little endogenous FVIII activity in patients administered emicizumab. The drastic shortening of aPTT due to emicizumab treatment was exaggerated in samples evaluated using OSA. Even at low emicizumab levels, out-of-range values were obtained in OSA with FVIII activity exceeding 150%.
Evaluation of the emicizumab-treated patient samples for FVIII inhibitors using ACL TOP OSA provided negative results except for one, while all CN-6000 CSA results were positive (Table 1). The interference of emicizumab in OSA can cause false-negative results. The false negativity of inhibitors may cause confusion regarding the patient’s clinical symptoms and result in delayed treatment. Therefore, it is particularly important to use bovine components that are not affected by emicizumab when testing samples from patients receiving emicizumab for FVII inhibitors using CSA.
The FVIII CSA demonstrated good analytical performance for use in clinical laboratories. Particularly, it is essential to measure endogenous or recombinant FVIII activity and FVIII inhibitors with CSA for emicizumab-treated patients. This is the first Korean study to measure FVIII activity and FVIII inhibitors by CSA using clinical samples.
Notes
REFERENCES
1. Shima M, Hanabusa H, Taki M, Matsushita T, Sato T, Fukutake K, et al. 2016; Factor VIII-mimetic function of humanized bispecific antibody in hemophilia A. N Engl J Med. 374:2044–53. DOI: 10.1056/NEJMoa1511769. PMID: 27223146.

2. Bowyer A, Kitchen S, Maclean R. 2020; Effects of emicizumab on APTT, one-stage and chromogenic assays of factor VIII in artificially spiked plasma and in samples from haemophilia A patients with inhibitors. Haemophilia. 26:536–42. DOI: 10.1111/hae.13990. PMID: 32249990.

3. Administration FaD. HEMLIBRA (emicizumab-kxwh) injection for subcutaneous use, prescribing information. In: Administration FaD, ed. Initial U.S.;2017.
4. Jenkins PV, Bowyer A, Burgess C, Gray E, Kitchen S, Murphy P, et al. 2020; Laboratory coagulation tests and emicizumab treatment A United Kingdom Haemophilia Centre Doctors' Organisation guideline. Haemophilia. 26:151–5. DOI: 10.1111/hae.13903. PMID: 31859415.

5. Nougier C, Jeanpierre E, Ternisien C, Proulle V, Hezard N, Pouplard C, et al. 2020; Emicizumab treatment: impact on coagulation tests and biological monitoring of haemostasis according to clinical situations (BIMHO group proposals). Eur J Haematol. 105:675–81. DOI: 10.1111/ejh.13490. PMID: 32668090.

Fig. 1
Regression graphs and Bland–Altman plots of FVIII assay: (A, B) for ACL TOP OSA vs. CN-6000 OSA, (C, D) for ACL TOP OSA vs. CN-6000 CSA, and (E, F) for CN-6000 OSA vs. CN-6000 CSA in the hemophilia patient group (excluding samples receiving non-factor treatment).
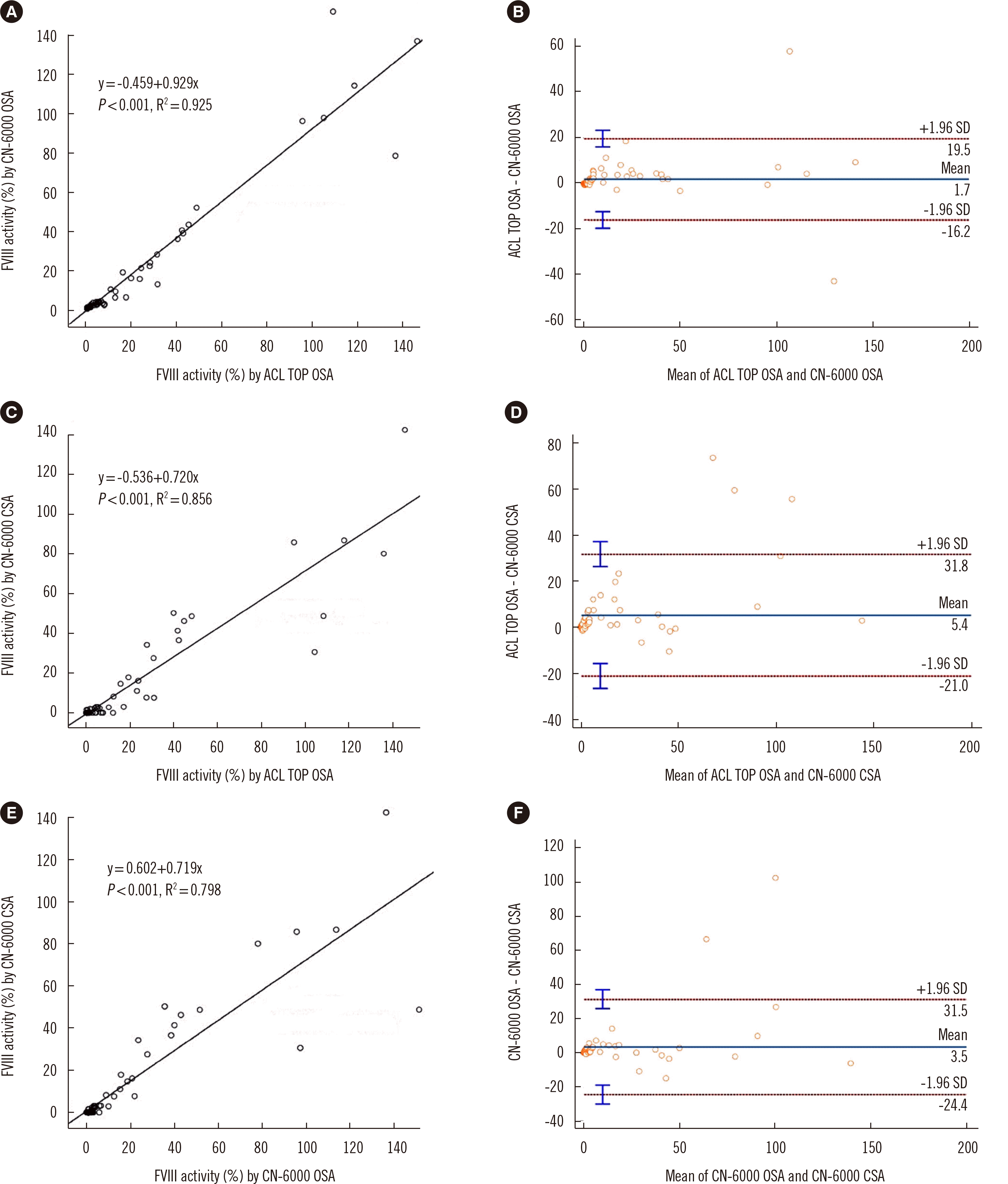
Table 1
FVIII activity and FVIII-inhibitor levels in patients with hemophilia treated with emicizumab, determined using three different assay/instrument combinations




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download