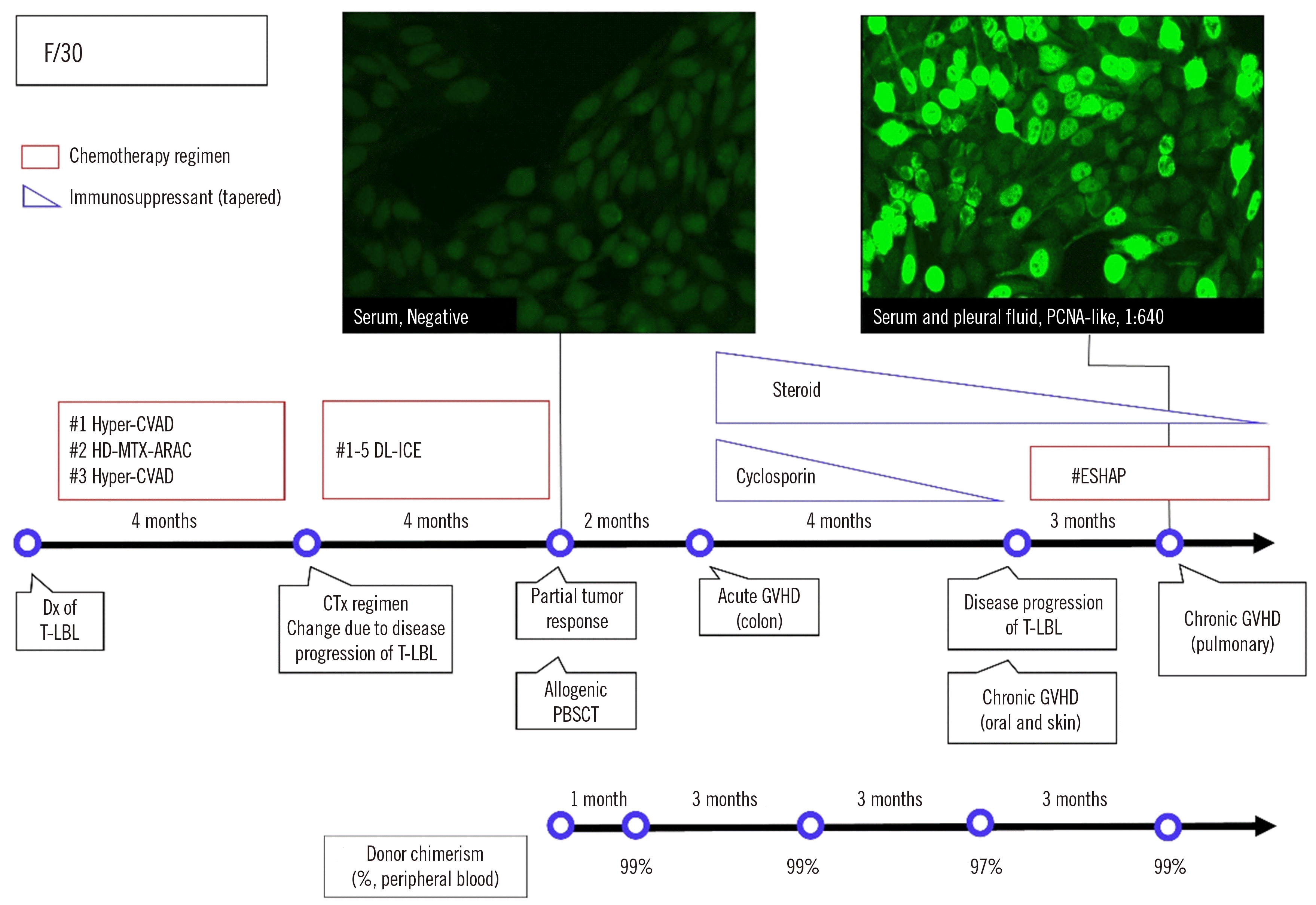
 | Fig. 1Timeline of the patient’s diagnosis and treatment history with representative ANA test images. The IIF test using Hep2 cells revealed a PCNA-like pattern. The small dots in the negative ANA test may be due to weakly reactive cytoplasmic antibodies or nonspecific antibodies against Hep2 substrates.
Abbreviations: PCNA, proliferating cell nuclear antigen; Dx, diagnosis; CTx, chemotherapy; PBSCT, peripheral blood stem cell transplantation; GVHD, graft-versus-host disease; CVAD, cyclophosphamide/doxorubicin/vincristine/dexamethasone; HD-MTX-ARAC, high-dose methotrexate and cytarabine; DL-ICE, dexamethasone/L-asparaginase/ifosfamide/carboplatin/etoposide; ESHAP, etoposide/methylprednisolone/cytarabine/cisplatin; ECT, etoposide/cyclophosphamide/total body irradiation.
|
Dear Editor,
Chronic graft-versus-host disease (cGVHD) is a multisystem immunological complication of allogeneic peripheral blood stem cell transplantation (PBSCT) and a major cause of late non-relapse mortality [1]. Like in novel autoimmune diseases, dysregulated B cell immunity is associated with autoantibody detection in cGVHD [1]. However, the clinical significance of autoantibodies in cGVHD and their relationship remain unclear [2]. Among the various autoantibodies, antinuclear antibodies (ANA) are the most commonly observed after PBSCT. ANA-nucleolar is the predominant pattern found in cGVHD patients after hematopoietic SCT [3, 4]. There is only one report on ANA-positivity with a proliferative cell nuclear antigen (PCNA)-like pattern in a cGVHD patient, and the clinical characteristics were not described in detail [4]. Here, we present a case of de novo antibody formation against PCNA in the blood and pleural fluid of a patient with systemic cGVHD. The Institutional Review Board of Seoul St. Mary’s Hospital. Seoul, Korea, approved this study (KC22ZISI0036) and waived the need for informed consent.
In May 2020, a 30-year-old woman was diagnosed with T-lymphoblastic lymphoma (T-LBL) involving a mediastinal mass at Seoul St. Mary’s Hospital. The patient underwent allogeneic PBSCT from an HLA-matched sibling donor in February 2021. The patient had no history of autoimmune disorders. An ANA test performed one week before PBSCT by indirect immunofluorescence (IIF) of Hep-2 cells was negative. A timeline of the patient’s diagnosis, treatment history, and ANA results is shown in Fig. 1.
During a follow-up in April 2021, the patient complained of severe nausea and vomiting, and an endoscopic biopsy revealed acute GVHD of the colon. In September 2021, oral and skin GVHD symptoms appeared, and chest computed tomography revealed progression of T-LBL. In December 2021, the patient showed exertional dyspnea with culture-negative pleural effusion and pulmonary function test (PFT) impairment and was clinically diagnosed as having pulmonary cGVHD.
IIF-ANA tests on serum and pleural fluid conducted to investigate pulmonary cGVHD showed strong positivity with a PCNA-like pattern with a 1:640 titer (Fig. 1). The results of enzyme immunoassays for anti-dsDNA, anti-cardiolipin, and anti-beta-2 GPI were negative. To confirm the presence of anti-PCNA antibody, a line immunoassay (LIA) using ANA profile 3 (Euroimmun, Oberlausitz, Germany), which detects antibodies against 15 antigens (nRNP/Sm, Sm, SS-A, Ro-52, SS-B, Scl-70, PM-Scl, Jo-1, CENP B, PCNA, dsDNA, nucleosomes, histones, ribosomal P-protein, and AMA-M2), was performed [5]. The LIA results were negative for all 15 antigens. This finding is similar to a previous finding where only 35% of serum samples showing a typical PCNA pattern on IIF staining were found positive in LIAs [6].
ANA has been detected in patients with non-Hodgkin’s lymphoma [7]. Thus, there was a possibility of aberrant ANA expression due to T-LBL in our case. However, our patient tested negative for ANA prior to PBSCT. Considering ANA expression was observed nine months after PBSCT in the clinical context of systemic GVHD symptoms, ANA positivity is more likely to be associated with GVHD than with T-LBL.
The clinical significance of autoantibodies, including ANA, in the development of cGVHD after HSCT remains controversial due to conflicting data across studies [2-4]. Therefore, the value of ANA as a predictive biomarker for cGVHD requires further evaluation. Nevertheless, other autoantibodies were not detected in our case, and there was a temporal relationship between the patient’s systemic GVHD expression and PCNA antibody detection. Therefore, we suggest a possible association between ANA-targeting PCNA and GVHD development.
Anti-PCNA antibodies are observed in 2%–6% of patients with systemic lupus erythematosus and in <5% of those with other autoimmune diseases [8]. An association between PCNA antibody and pulmonary disease has been reported, but there was no evidence of autoimmune disease [9]. These results suggest that the presence of anti-PCNA antibodies has a low specificity for certain disease entities. Our unique case indicates that the ANA-reactivity of a PCNA-like pattern can be observed in patients with cGVHD after PBSCT.
Given that high PCNA-antibody titers were detected in the patient’s serum and pleural fluid when her respiratory symptoms occurred, we postulate that the development of pulmonary cGVHD was possibly associated with the PCNA antibodies. Pulmonary complications significantly contribute to late mortality after PBSCT, and noninfectious pulmonary complications are strongly associated with cGVHD. Pulmonary cGVHD can present as bronchiolitis obliterans, with disease severity ranging from subclinical PFT impairment to respiratory failure [10]. Because of the nonspecific clinical symptoms, a standardized approach is required to detect pulmonary cGVHD before irreversible structural changes occur [10]. As seen in our case, the detection of autoantibodies may help in the diagnosis of pulmonary cGVHD when the patient shows bronchiolitis obliterans symptoms in the absence of infectious causes.
Comprehensive clinical and immunological evaluations of additional cases are required to clarify the significance of anti-PCNA autoantibodies in cGVHD and their relationship.
Go to : 
Notes
AUTHOR CONTRIBUTIONS
Oh EJ and Kang H designed the study; SG Cho provided the clinical data; Kang H contributed to data acquisition; Oh EJ and Kang H analyzed and interpreted the data; Kang H wrote the manuscript; and Oh EJ edited the manuscript. All authors have read and agreed to the final version of the manuscript.
Go to : 
REFERENCES
1. Min CK. 2011; The pathophysiology of chronic graft-versus-host disease: the unveiling of an enigma. Korean J Hematol. 46:80–7. DOI: 10.5045/kjh.2011.46.2.80. PMID: 21747879. PMCID: PMC3128905.

2. Kuzmina Z, Gounden V, Curtis L, Avila D, Rnp Taylor T, Baruffaldi J, et al. 2015; Clinical significance of autoantibodies in a large cohort of patients with chronic graft-versus-host disease defined by NIH criteria. Am J Hematol. 90:114–9. DOI: 10.1002/ajh.23885. PMID: 25363867. PMCID: PMC5535796.

3. Patriarca F, Skert C, Sperotto A, Zaja F, Falleti E, Mestroni R, et al. 2006; The development of autoantibodies after allogeneic stem cell transplantation is related with chronic graft-vs-host disease and immune recovery. Exp Hematol. 34:389–96. DOI: 10.1016/j.exphem.2005.12.011. PMID: 16543073.

4. Hao B, Gao S, Sang YW, Wang L, Meng XQ, You JY. 2019; Potential value of autoantibodies as biomarkers of chronic graft-versus-host disease after allogeneic stem cell transplantation. J Zhejiang Univ Sci B. 20:849–60. DOI: 10.1631/jzus.B1900205. PMID: 31489804. PMCID: PMC6751484.

5. Yoon S, Moon H, Kim H, Hur M, Yun Y. 2022; Clinical performance of two automated immunoassays, EliA CTD Screen and QUANTA Flash CTD Screen Plus, for antinuclear antibody screening. Ann Lab Med. 42:63–70. DOI: 10.3343/alm.2022.42.1.63. PMID: 34374350. PMCID: PMC8368234.

6. Mahler M, Silverman ED, Fritzler MJ. 2010; Novel diagnostic and clinical aspects of anti-PCNA antibodies detected by novel detection methods. Lupus. 19:1527–33. DOI: 10.1177/0961203310375265. PMID: 20647252.

7. Guyomard S, Salles G, Coudurier M, Rousset H, Coiffier B, Bienvenu J, et al. 2003; Prevalence and pattern of antinuclear autoantibodies in 347 patients with non-Hodgkin's lymphoma. Br J Haematol. 123:90–9. DOI: 10.1046/j.1365-2141.2003.04587.x. PMID: 14510947.

8. Mahler M, Miyachi K, Peebles C, Fritzler MJ. 2012; The clinical significance of autoantibodies to the proliferating cell nuclear antigen (PCNA). Autoimmun Rev. 11:771–5. DOI: 10.1016/j.autrev.2012.02.012. PMID: 22381917.

9. Lee ZY, Think-You K. 2014; A case of small cell lung carcinoma with coexisting autoantibodies against proliferating cell nuclear antigen and centriole detected using the autoimmune target test. Lab Med Online. 4:218–21. DOI: 10.3343/lmo.2014.4.4.218.

10. Hildebrandt GC, Fazekas T, Lawitschka A, Bertz H, Greinix H, Halter J, et al. 2011; Diagnosis and treatment of pulmonary chronic GVHD: report from the consensus conference on clinical practice in chronic GVHD. Bone Marrow Transplant. 46:1283–95. DOI: 10.1038/bmt.2011.35. PMID: 21441964. PMCID: PMC7094778.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download