Dear Editor,
The Philadelphia chromosome (Ph) results from the reciprocal translocation t(9;22)(q34.1;q11.2) and comprises the BCR-ABL1 fusion [1,2]. In B lymphoblastic (B)-ALL/lymphoblastic lymphoma (LBL), Ph+ cases account for ~25% of adult cases and 2%–4% of pediatric cases. It is associated with poor prognosis and requires therapy with tyrosine kinase inhibitors (TKIs) [3]. In contrast, Ph+ T-ALL/LBL is so rare that the clinical relevance, prognostic significance, and treatment approaches are not clearly defined for these patients [1, 4, 5].
STIL-TAL1 fusion is identified in 9%–26% of T-ALL cases and results from interstitial deletion between the 5´ untranslated region of TAL1 and STIL, located on chromosome 1p33. This condition can promote T-cell leukemogenesis due to TAL1 overexpression; however, its clinical significance is controversial [6].
We report a rare case of a STIL-TAL1-positive T-ALL patient who also carried a minor Ph+ clone. The Institutional Review Board of Severance Hospital, Seoul, Korea, approved this study (4-2022-0305) and waived the need for informed consent.
An 11-year-old boy with no significant medical history presented with a cervical mass. He underwent cervical lymph node biopsy at a tertiary hospital in February 2022; the pathologic diagnosis was suggestive of precursor T-cell lymphoid neoplasm. The patient was referred to our hospital for further evaluation and was admitted to the isolation ward after testing positive for coronavirus disease (COVID-19).
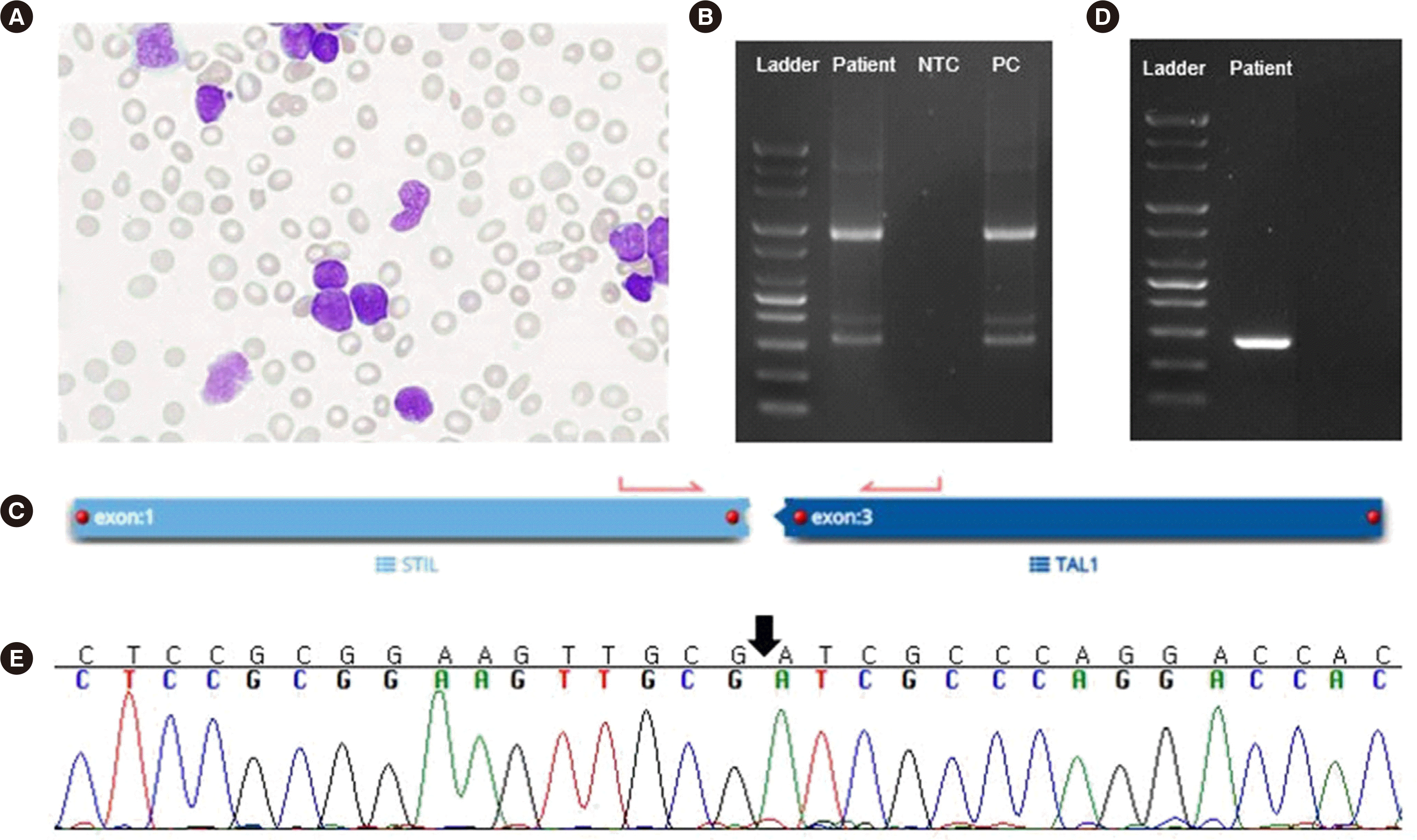
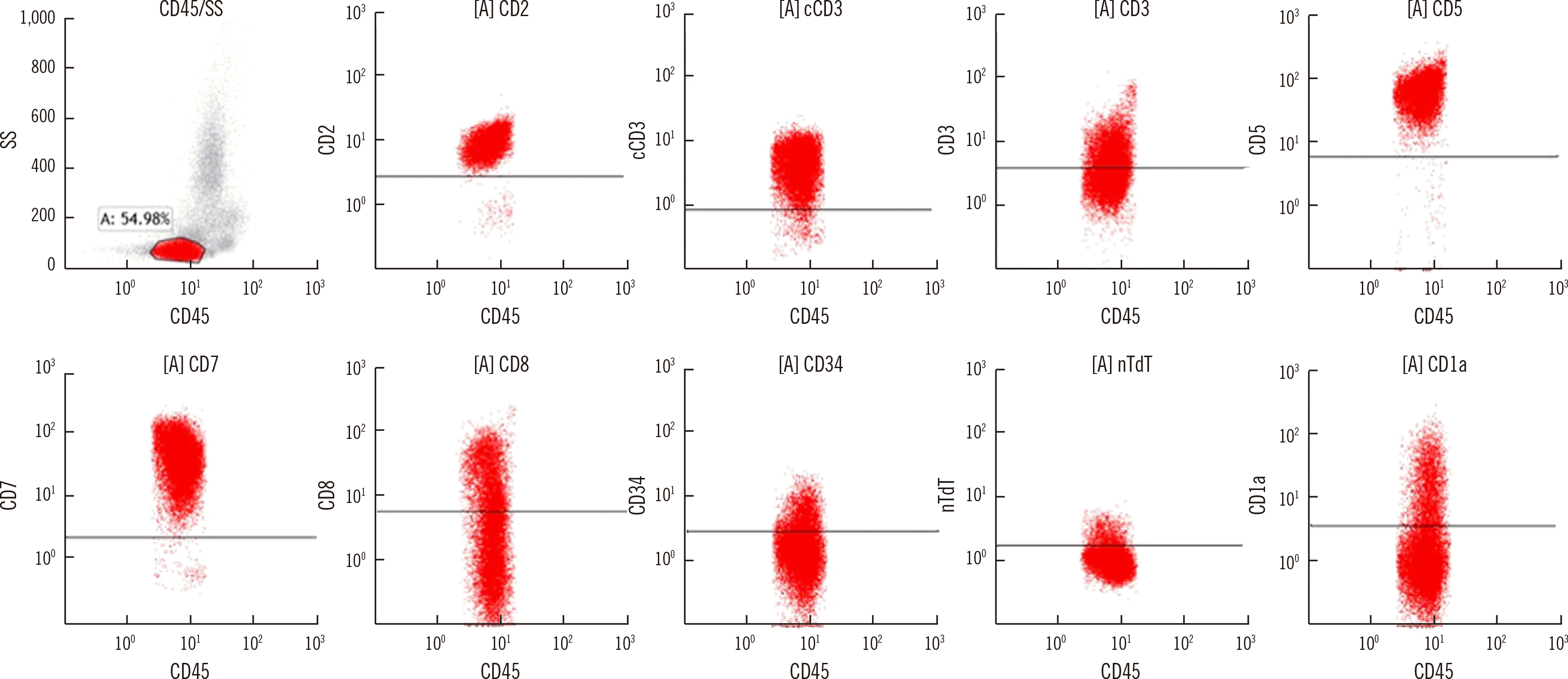
Complete blood count was white blood cell (WBC) count, 252.55×109/L; hemoglobin, 10.3 g/dL; platelet count, 47×109/L. Peripheral blood smear showed 89% blasts (Fig. 1A). Because of COVID-19, initial bone marrow examination was not performed; the entire diagnostic work-up was conducted using peripheral blood samples. Chromosome analysis results were not interpretable because of a lack of metaphase cells. Flow cytometry showed an abnormal leukemic population, consistent with T-ALL/LBL (Fig. 2). Reverse transcription (RT)-PCR using total RNA isolated from WBCs (HemaVision kit; DNA-Diagnostic, Risskov, Denmark) revealed an e1a2 rearrangement (Fig. 1B). Quantitative RT-PCR (Ipsogen BCR-ABL1 mbcr kit; Qiagen, Hilden, Germany) revealed a BCR-ABL1-to-ABL1 transcript ratio of 6.11×10–4. RNA fusion panel (FusionPlex Pan-Heme Panel, ArcherDX, CO, USA) detected a STIL-TAL1 rearrangement (Fig. 1C).
To confirm the STIL-TAL1 transcript, RT-PCR and direct sequencing were performed (Fig. 1D, E). Next-generation sequencing revealed a copy number loss of CDKN2A/B and two frameshift mutations of PTEN exon 7 in trans (p.Leu247LysfsTer11, 11.3% variant allele frequency [VAF] and p.Leu247- HisfsTer14, 43.7% VAF), which are frequently associated with T-ALL [7]. A TCR gene clonality assay (LymphoTrack; Invivoscribe Technologies, San Diego, CA, USA) showed clonal rearrangement in TCRB (Db1/Jb1-2; 40.1% of total reads) and TCRG (Vg3/none; 40.8% of total reads).
In our case, BCR-ABL1 fusion was detected only by RT-PCR, not by RNA fusion panel analysis. The reason for this discrepancy maybe the different levels of sensitivity of the two tests [8]. Based on a previous report describing cases of T-ALL with a minor Ph+ clone [9], our case can be considered STIL-TAL1-positive T-ALL with a minor Ph+ clone. The patient received induction therapy and achieved complete remission with incomplete hematologic recovery. He continued treatment with nelarabine, and hematopoietic stem cell transplantation is planned after consolidation therapy with the addition of imatinib.
Ph+ T-ALL/LBL is extremely rare, and only a few cases have been reported [1, 4, 5]. Furthermore, Ph+ T-ALL with STIL-TAL1 fusion has been reported only once [10]. To our knowledge, this is the first case of Ph+ T-ALL as well as of Ph+ T-ALL with STIL-TAL1 fusion reported in Korea.
Because of its rarity, the clinical significance of Ph+ clones in Ph+ T-ALL is not clearly defined. However, as expression of the BCR-ABL1 oncogene provides proliferative and survival advantages, there may be risk of clonal selection of Ph+ clones, possibly contributing to the worsening of the prognosis. This can be implied from a Ph+ T-ALL case in which disease progression was associated with a higher BCR-ABL1 transcript level and an increase in proportion of BCR-ABL1-positive cells, indicating the contribution of Ph+ clones in disease progression, leading to a poor outcome [1, 11]. As for the treatment approach of Ph+ T-ALL, the addition of TKIs to chemotherapy should be considered as cases in which patients showed good responses to TKIs have been reported [9].
We report a rare case of STIL-TAL1-positive T-ALL with a minor Ph+ clone. More extensive studies are needed to assess the true incidence of T-ALL with a minor Ph+ clone and its clinical significance. Moreover, research on the role of these coexisting rearrangements in leukemogenesis is warranted. Clinical laboratories should be aware of this rare phenomenon and use caution when reporting patients’ test results.
Notes
AUTHOR CONTRIBUTIONS
Koo YK collected patient data and wrote the manuscript. Kim H performed the genetic and flow cytometric analyses. Shin S supervised the research and reviewed and edited the manuscript. All authors reviewed and approved the final version of the manuscript.
REFERENCES
1. Li X, Ping N, Wang Y, Xu X, Gao L, Zeng Z, et al. 2020; Case report: a case with Philadelphia chromosome positive T-cell lymphoblastic lymphoma and a review of literature. Front Oncol. 10:584149. DOI: 10.3389/fonc.2020.584149. PMID: 33552960. PMCID: PMC7857119.

2. Choi H, Cho SR, Yang D, Lee W, Hwang H, Lee HS, et al. 2020; A Case of Preleukemic Chronic Myeloid Leukemia Following Chemotherapy and Autologous Transplantation for T-lymphoblastic Lymphoma. Ann Lab Med. 40:417–20. DOI: 10.3343/alm.2020.40.5.417. PMID: 32311856. PMCID: PMC7169630.

3. Swerdlow SH, Campo E, editors. WHO Classification of tumours of haematopoietic and lymphoid tissues. 4th ed. WHO classification of tumours;2017. volume 2.
4. Raanani P, Trakhtenbrot L, Rechavi G, Rosenthal E, Avigdor A, Brok-Simoni F, et al. 2005; Philadelphia-chromosome-positive T-lymphoblastic leukemia: acute leukemia or chronic myelogenous leukemia blastic crisis. Acta Haematol. 113:181–9. DOI: 10.1159/000084448. PMID: 15870488.

5. Jain P, Kantarjian H, Jabbour E, Kanagal-Shamanna R, Patel K, Pierce S, et al. 2017; Clinical characteristics of Philadelphia positive T-cell lymphoid leukemias-(De novo and blast phase CML). Am J Hematol. 92:E3–4. DOI: 10.1002/ajh.24579. PMID: 27727470. PMCID: PMC5551407.

6. D'Angiò M, Valsecchi MG, Testi AM, Conter V, Nunes V, Parasole R, et al. 2015; Clinical features and outcome of SIL/TAL1-positive T-cell acute lymphoblastic leukemia in children and adolescents: a 10-year experience of the AIEOP group. Haematologica. 100:e10–3. DOI: 10.3324/haematol.2014.112151. PMID: 25304610. PMCID: PMC4281327.
7. Furness CL, Mansur MB, Weston VJ, Ermini L, van Delft FW, Jenkinson S, et al. 2018; The subclonal complexity of STIL-TAL1+ T-cell acute lymphoblastic leukaemia. Leukemia. 32:1984–93. DOI: 10.1038/s41375-018-0046-8. PMID: 29556024. PMCID: PMC6127084.

8. Kim B, Lee H, Shin S, Lee ST, Choi JR. 2019; Clinical evaluation of massively parallel RNA sequencing for detecting recurrent gene fusions in hematologic malignancies. J Mol Diagn. 21:163–70. DOI: 10.1016/j.jmoldx.2018.09.002. PMID: 30347268.

9. Prebet T, Mozziconacci MJ, Sainty D, Arnoulet C, Lafage M, Dastugue N, et al. 2009; Presence of a minor Philadelphia-positive clone in young adults with de novo T-cell ALL. Leuk Lymphoma. 50:485–7. DOI: 10.1080/10428190802601148. PMID: 19197723.
10. Chen X, Wang F, Zhang Y, Wang M, Tian W, Teng W, et al. 2018; Retrospective analysis of 36 fusion genes in 2479 Chinese patients of de novo acute lymphoblastic leukemia. Leuk Res. 72:99–104. DOI: 10.1016/j.leukres.2018.08.009. PMID: 30125757.

11. Tchirkov A, Bons JM, Chassagne J, Schoepfer C, Kanold J, Briançon G, et al. 1998; Molecular detection of a late-appearing BCR-ABL gene in a child with T-cell acute lymphoblastic leukemia. Ann Hematol. 77:55–9. DOI: 10.1007/s002770050412. PMID: 9760154.

Fig. 1
Morphological and genetic analysis. (A) Blasts on a peripheral blood smear (Wright–Giemsa stain, 1,000×). (B) Agarose gel electrophoresis of the reverse transcription (RT)-PCR product showing the e1a2 (p190) BCR-ABL1 transcript (HemaVision kit; DNA-Diagnostic, Risskov, Denmark). (C) STIL-TAL1 fusion detected using an RNA fusion panel (FusionPlex Pan-Heme Panel; ArcherDX, Boulder, CO, USA). (D) RT-PCR using cDNA and subsequent (E) Sanger sequencing confirmed breakpoints in exons 1 and 3 of STIL and TAL1, respectively.
Abbreviations: NTC, non-template control; PC, positive control.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download