This article has been
cited by other articles in ScienceCentral.
Abstract
Sclerosing polycystic adenosis (SPA) is a rare, asymptomatic disease that occurs mainly in the salivary glands. We report the case of a 51-year-old man who presented with trismus and pain upon mouth opening. Magnetic resonance imaging revealed a 2-cm mass located in the anterior portion of the left parotid gland. SPA was diagnosed based on histopathological examination of the surgical specimen. In pathologic findings, there was a well-circumscribed multicystic nodule in the parenchyma. Dense fibrosis and chronic non-specific inflammatory cells were observed in the stroma. In 13 previous reports on SPA, the most preferred treatment was superficial or total parotidectomy. This report suggests that simple excision of SPA preserves facial nerve function and facial volume.
Go to :

Keywords: Neoplasm, Benign neoplasm, Tumor, Parotid gland, Trismus
I. Introduction
Sclerosing polycystic adenosis (SPA) is a rare, usually asymptomatic, lesion of the salivary gland. It is considered a reactive, inflammatory disease and is mostly observed in the parotid gland (80%)
1. Although SPA has characteristic features on histopathologic examination, including multiple lobules with sclerosing and hyalinized connective tissue, dilated cystic-like ducts, and ductal and acinar hyperplasia, it is difficult to diagnose clinically even with computed tomography, magnetic resonance imaging (MRI), and fine-needle aspiration biopsy (FNAB). The World Health Organization classified SPA as a benign, non-neoplastic epithelial tumor in 2018
2; however, controversies regarding its possible malignancy remain. Further, previously reported cases of SPA were not accompanied by limited mouth opening. This article reports a case of a patient with SPA accompanied by acute trismus, which recovered after surgery.
Go to :

II. Case Report
A 51-year-old man presented with left preauricular area swelling of 3-4 years’ duration. The patient had no other medical history besides being previously diagnosed with chronic hepatitis B. Initially, the swelling experienced by the patient was painless; it gradually became painful, and the patient developed limited mouth opening. Upon visiting a hospital, physical examination revealed swelling of the left preauricular area with induration tendency and tenderness upon palpation. The patient’s maximal mouth opening was 35 mm and was accompanied by pain in the left preauricular area.
MRI revealed a 2-cm lobulated mass in the anterior portion of the left parotid gland. The mass was hyperintense on T2-weighted imaging and showed rim enhancement on a gadolinium-enhanced image. Local enhancement was found along the border of the mass.(
Fig. 1) This finding led to the suspicion of a benign salivary gland tumor with cystic degeneration, such as a Warthin tumor. The tumor severely compressed the masseter muscle anteriorly, which was suspected to be related to the patient’s trismus.
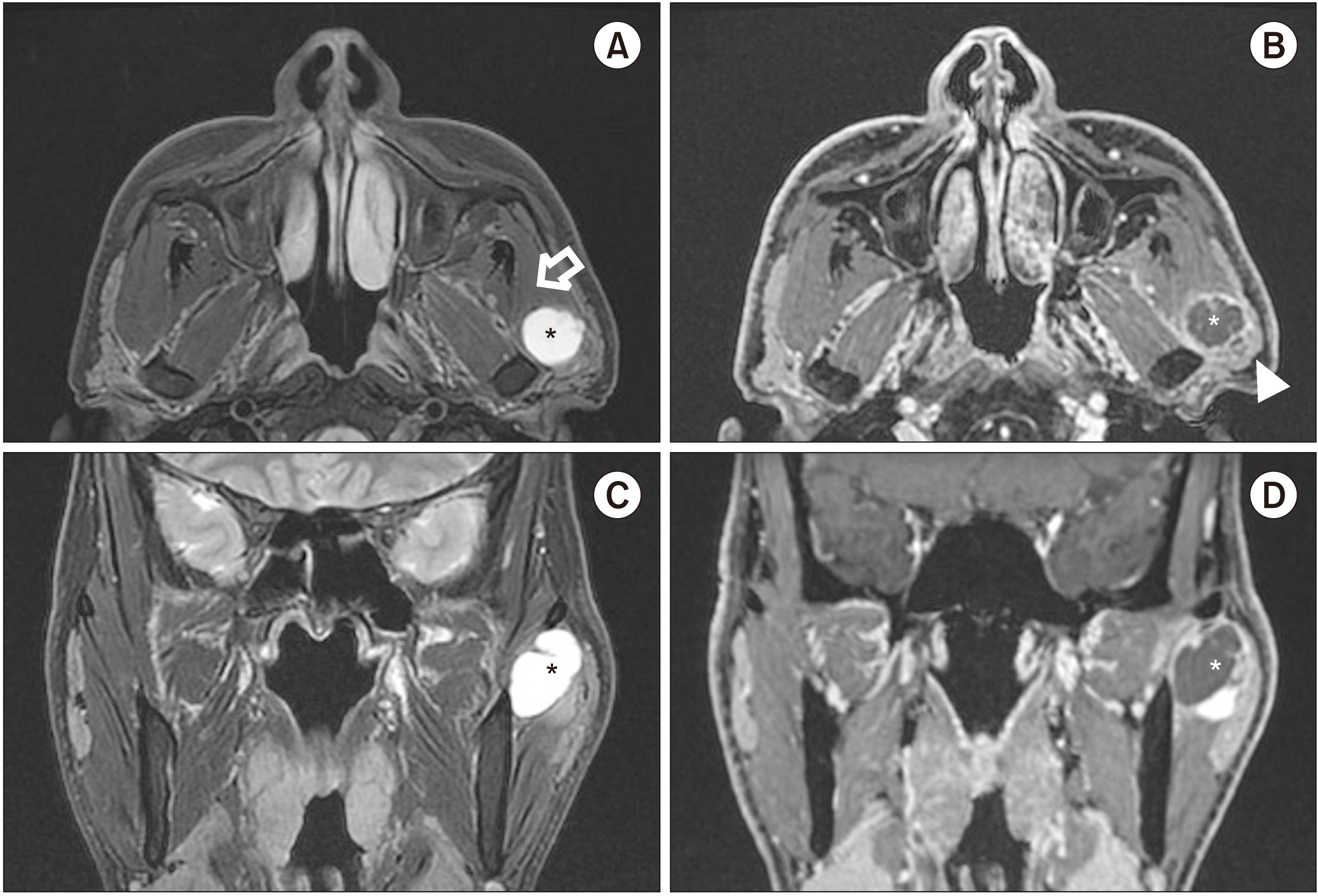 | Fig. 1Magnetic resonance imaging revealed a 2-cm-sized mass in the anterior portion of the left parotid gland, compressing the masseter muscle anteriorly (A). The lesion is mainly T2-hyperintense; it is a rim-enhancing lesion with a small localized enhancing area (A, C). The lesion in T1-hypointense (B, D). (Asterisks: cystic area, Hollow arrow: masseter muscle, Arrowhead: solid area of the tumor) 
|
Although superficial parotidectomy was planned, a cyst-like mass was identified beneath the facial nerve. The parotid gland was repositioned after enucleation to decrease facial nerve weakness and preserve facial volume. After enucleation, the mass was 13 mm×5 mm×5 mm.
Histopathological examination of the surgical specimen revealed a nodular mass embedded within the parotid glandular parenchyma, showing multicystic changes.(
Fig. 2. A) The multicystic nodule was well-circumscribed, but not encapsulated. Cystic dilatation of the salivary gland duct and tubuloacinar hyperplasia was observed.(
Fig. 2. B-D) Furthermore, there were dense fibrosis and chronic non-specific inflammatory cells within the stroma.(
Fig. 2. B, 2. C) A hyalinized nodule surrounded the atrophic duct, and some acini and ducts showed prominent eosinophilic cytoplasmic changes.(
Fig. 2. C) Further, there was sebaceous metaplasia of the glandular duct.(
Fig. 2. D) Considering the degenerative and reactive changes caused by inflammation, a final diagnosis of SPA was established.
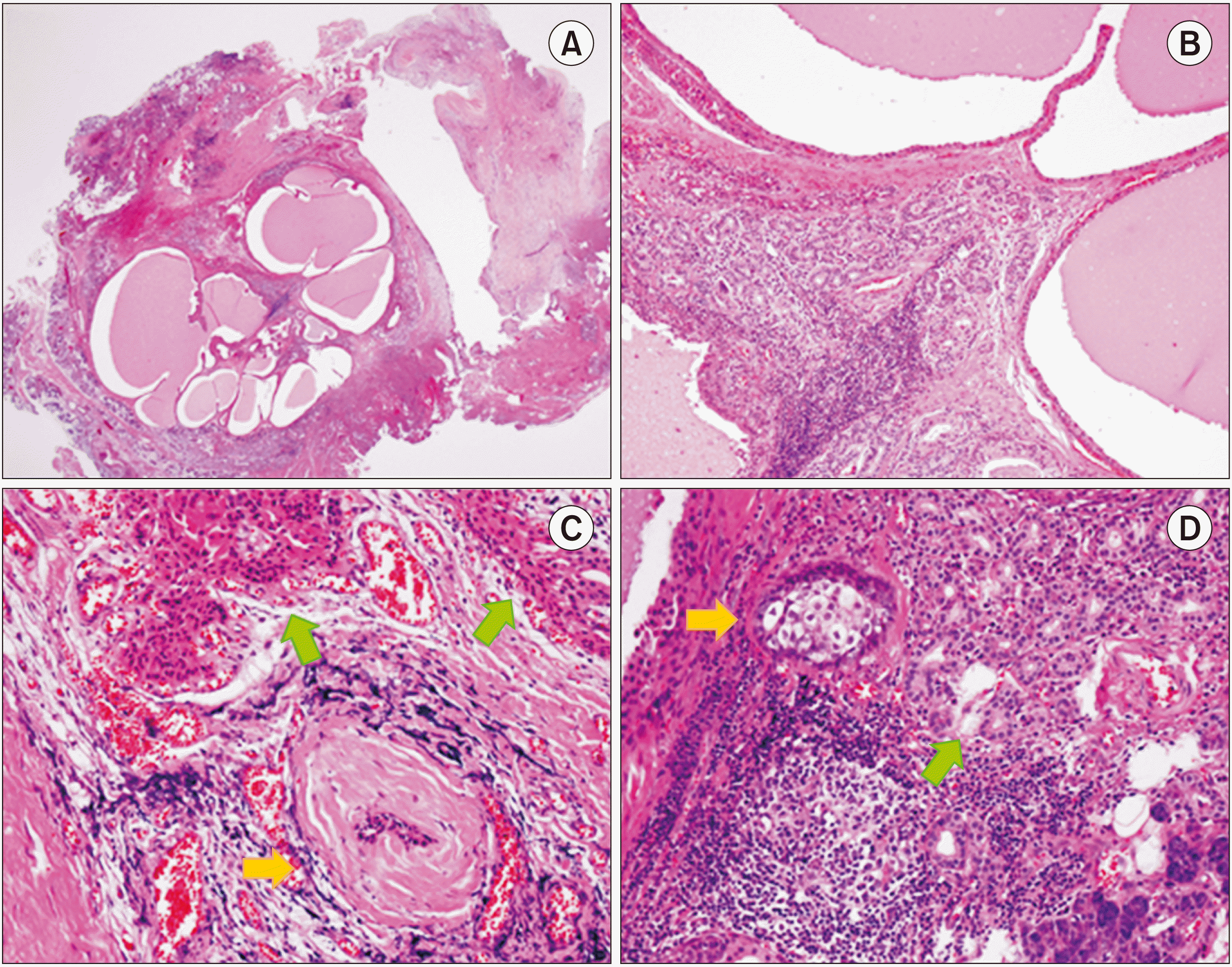 | Fig. 2Histopathological examination of the surgical specimen through H&E staining. (A). A multicystic nodular mass within the parotid parenchyma was observed under low magnification. Original magnification, ×12.5. (B). The cysts lining the epithelium show simple or stratified cuboidal cells with oncocytic cytoplasm. Ductal hyperplasia, chronic non-specific inflammation, and dense fibrosis are present. Original magnification, ×100. (C). A characteristic hyalinized nodule surrounding the degenerative duct is observed (yellow arrow). Some acini and ducts show eosinophilic cytoplasmic changes (green arrows). Original magnification, ×200. (D). Some glandular ducts show sebaceous metaplasia (yellow arrow). Prominent lymphocytic infiltration and ductal hyperplasia are also observed (green arrow). Original magnification, ×200. 
|
After excision, maximal mouth opening within the normal range was recovered. The mouth opening was 50 mm at the time of suture removal and 56 mm at three months after surgery. Follow-up MRI performed after six months revealed no evidence of recurrence.
Go to :

III. Discussion
Thirteen studies have reported the presence of SPA in the parotid gland (
Table 1)
3-14; all patients underwent total or superficial parotidectomy. The common clinical feature was a slowly growing and palpable but not painful SPA. No case with trismus was observed in these studies. Moreover, no recurrence was noted during a five-month to three-year follow-up.
Table 1
Literature review on sclerosing polycystic adenosis of the parotid gland
|
Study |
Patient age (yr)/ sex (M/F) |
Presenting features |
Treatment |
Recurrence |
Follow-up period |
|
Espinosa et al.3 (2017) |
64/F |
A 3-cm well defined, ovoid, mobile, and slightly tender lump located in the inferior portion of the right parotid gland |
SP |
Not described |
Not described |
|
77/F |
A painless, 2-cm well circumscribed parotid tumor that was not attached to the overlying skin |
TP |
Not described |
Not described |
|
Bharadwaj et al.4 (2007) |
45/F |
A pea-sized lump behind the left angle of the mandible |
SP |
None |
3 yr |
|
Eliot et al.5 (2012) |
25/F |
A 1-year history of painless swelling in the right parotid gland |
TP |
Not described |
Not described |
|
Tang et al.6 (2016) |
74/F |
A 4-year history of a growing, painful, left-sided neck mass |
TP+SND (level II) |
Not described |
Not described |
|
Kim et al.7 (2012) |
47/M |
A long-standing asymptomatic mass located in the left parotid gland |
Partial parotidectomy of the lower portion of the left parotid gland |
None |
5 mo |
|
Perottino et al.8 (2010) |
68/M |
An isolated, mobile, pain-free, juxta-lobular 2-cm-diameter mass that is firm on palpation |
Conservative subtotal parotidectomy |
None |
1 yr |
|
Kawai et al.9 (2019) |
12/M |
Painless swelling of the right neck |
TP |
Not described |
Not described |
|
Petersson et al.10 (2011) |
45/F |
An asymptomatic nodule |
SP |
Not described |
Not described |
|
Matsumoto et al.11 (2016) |
33/M |
A palpable, painless, and elastic hard mass in the left preauricular area without facial nerve paralysis |
SP |
None |
28 mo |
|
Kahraman et al.12 (2020) |
35/F |
A slow-growing, painless, hard parotid mass, noticed recently |
TP |
Not described |
Not described |
|
Tokyol et al.13 (2012) |
77/F |
A slow-growing mass |
SP |
None |
1 yr |
|
Fulciniti et al.14 (2010) |
57/M |
A long-standing ovoid mass |
SP |
None |
5 mo |

MRI did not provide sufficient information for a diagnosis of SPA. Most previous reports characterized SPAs as lobular with T1 hypointensity and T2 hyperintensity on imaging. These imaging features are not common, and any type of benign salivary gland tumor, such as pleomorphic adenoma or Warthin tumor, can be considered in the differential diagnosis. FNAB has also been ineffective in the diagnosis of SPA, which was diagnosed in only one of 30 cases. This was presumed to be due to the difficulty in aspirating a focal solid mass, since SPA is mostly composed of cystic structures.
Histopathologic examination with hematoxylin and eosin staining is considered effective for the diagnosis of SPA. SPA is unencapsulated but is typically well-circumscribed, with a sclerotic, hyalinized stroma. The ducts are usually dilated and show cystic changes. The ductal and acinar structures commonly display hyperplasia and apocrine-like metaplasia; sebaceous-like structures are rare. Gnepp et al.
15 described these structures as mucin-containing, squamous, sebaceous-like, ballooned cells. They also observed brightly eosinophilic, intracytoplasmic, and modified zymogen granules
15.
Skálová et al.
16 reported immunoreactivity of progesterone and estrogen receptors. They reported that most epithelial cells of the cystic ducts, some foam cells lining the ducts, and the cells within the dysplastic foci expressed strong nuclear immunoreactivity for progesterone receptors (approximately 80%). On the other hand, staining for estrogen receptors was detected in approximately 20% of ductal cells in dysplastic foci only. This suggests that hormonal stimulation significantly affects the pathogenesis of SPA
16.
Espinosa et al.
3 reported 36 patients with SPA in the parotid gland, of whom 61.1%, 22.2%, and 16.6% underwent superficial parotidectomy, total parotidectomy, and tumor enucleation, respectively. Gnepp et al.
15 also reported 13 patients with SPA in the parotid gland, of whom ten (76.9%) and three (23.1%) patients underwent parotidectomy and tumor excision, respectively. SPA recurrence is rare and often attributed to incomplete surgical excision or the presence of multiple lesions
5,15,17. Complete excision and facial nerve preservation are most recommended for treatment of recurrent SPA. Additionally, SPA does not invade adjacent anatomical structures; hence, simple excision of the lesion is sufficient, and superficial or total parotidectomy should be considered for facial nerve preservation.
Whether SPA is a neoplasm and develops into a carcinoma remains controversial. SPA has been considered a reactive, inflammatory, pseudoneoplastic lesion
15,17. Skálová et al.
16 described three patients with SPA who had varying degrees of dysplastic change within the epithelium, one of whom met the criteria for ductal carcinoma
in situ. The apocrine subtype of salivary duct carcinoma is typically accompanied by apocrine differentiation, as is similarly observed in SPA. Phosphatidylinositol 3-kinase (PI3K) pathway mutations are commonly observed in salivary duct carcinoma. Bishop et al.
18 revealed that ductal and acinar cells in SPA lose phosphatase and tensin homolog (
PTEN) expression. PI3K pathway alteration with
PTEN mutations suggests the similarity between SPA and apocrine-type salivary duct carcinoma. Skálová et al.
19 surveyed six patients with SPA and found that cells of SPA are clonal. Therefore, it is evident that SPA is a neoplasm, not just a reactive, inflammatory disease.
SPA is a well-demarcated benign lesion, commonly misdiagnosed as Warthin tumor or pleomorphic adenoma. Although the literature describes the management of this lesion with superficial or total parotidectomy, simple excision is recommended considering its capability to prevent recurrence and preserve the facial nerve. Histologic examination is the standard method for diagnosis of SPA.
Go to :







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download