Abstract
Objectives
There have been few studies to date on the residual effect of bisphosphonate. This study investigated the radiographic changes of mandibular cortical thickness upon bisphosphonate drug holiday.
Materials and Methods
This retrospective study includes 36 patients diagnosed with MRONJ (medication-related osteonecrosis of the jaw) at Ajou University Dental Hospital in 2010-2021. All patients stopped taking bisphosphonate under consultation with the prescribing physicians. Panoramic radiographs were taken at the start of discontinuation (T0), 12 months after (T1), and 18 months after (T2) discontinuation of bisphosphonate, respectively. Mental index and panoramic mandibular index were calculated using Ledgerton’s method. Paired t-tests were used to analyze differences over time.
Medication-related osteonecrosis of the jaw (MRONJ) was first reported by Marx in 2003, and reports related to the disease have since gradually increased. Bisphosphonate drugs are widely used as a treatment for osteoporosis through the mechanism of inhibiting osteoclasts; they are also used for malignant tumors (i.e., multiple myeloma), hypercalcemia, and metastatic bone tumor.
MRONJ is a disease that can be diagnosed when bone is exposed in the oral cavity or there is a fistula that can be probed into bone for more than eight weeks despite proper management. In addition, there should be no history of radiation therapy or metastatic tumor in the skeleton.
The pathophysiology of MRONJ is multifactorial, but low bone turnover rate due to bisphosphonate treatment and pre-existing inflammation are sufficient for MRONJ diagnosis. While reduced bone remodeling due to bisphosphonate is expected to be the major cause, parathyroid hormone can help treat MRONJ by reversing reduced bone turnover1,2.
There is no consensus on whether MRONJ should be treated surgically or medically. Currently, conservative and surgical treatment are used interchangeably. The American Association of Oral and Maxillofacial Surgeons issued a revised position paper for MRONJ in 2022, recommending both conservative and surgical approaches for MRONJ patients3.
When it comes to treatment via drug holiday, controversy remains. Some authors recommend consulting with a physician to discontinue MRONJ-related drugs such as bisphosphonate or denosumab, until the lesion is cured to prevent exacerbation during the treatment process4. Others reported that drug holiday was not useful for preventing MRONJ. Meanwhile, it is still unknown whether or when bone density decreased after bisphosphonate discontinuation. If bone bone density decreases, we consider when it decreases might be the radiologic evidence of a bisphosphonate drug holiday. This study tries to answer these questions by investigating changes in mandibular cortical thickness.
The medical records of 277 patients diagnosed with MRONJ at Ajou University Hospital from January 2010 to May 2021 were analyzed. After we reviewed the records, only patients who discontinued bisphosphonate treatment and whose follow-up period was at least 12 months were selected. Demographics of the patients involved are described in Table 1. This study was conducted after approval from the Institutional Review Board of Ajou University Hospital (approval No. AJIRB-MED-MDB-21-538), and the informed consent was waived by the IRB.
After marking the position of the mental foramen on panoramic radiographs using the method of Ledgerton et al.5, the line (b) is drawn perpendicular to the tangent line (a) of the mandibular inferior border and crossing the center of the mental foramen. A PMI value is obtained by dividing the thickness of the mandibular cortex (C) by the distance from the mandibular border to either the upper or lower end of mental foramen (I and S)5.(Fig. 1) Thereby, we obtain the following equations:
PMI(I)=C/I
PMI(S)=C/S
MI is defined as the gap between the upper and lower tangent line of the mandibular cortex.(C in Fig. 1)
Panoramic X-rays (CS 8100, 73 kVp, 10 mA, 10.8 seconds; Careream Dental, Atlanta, GA, USA) were taken for each of the patients. Measurement was done using INFINITT PACS software (Infinitt Healthcare, Seoul, Korea). All measurements were performed at 100% and 150% magnification. The mandibular cortex line was marked on a 100%-magnified radiograph, and the indices (PMI and MI) for statistical comparison were measured on a 150%-magnified image. They were measured on either the left or right side of the patient. In patients who underwent sequestrectomy, followed by rhBMP-2 (recombinant human bone morphogenetic protein-2) regeneration, indices on the surgically treated area were excluded. As a result, only non-surgically treated areas of the mandible were selected in this study. After first measurement, another measurement was executed after a period time (at least 90 days) to evaluate the intra-observer reliability. Nine patients were randomly selected for re-measurement. Fifty-nine sets of data comparing 1st and 2nd measurement were produced.
Data from a total of 36 patients who met the inclusion criteria were used. For these patients, index values at the starting point of discontinuation (T0), 12 months after discontinuation (T1), and 18 months after discontinuation (T2) were compared. Not all 36 patients underwent radiographic examination at the exact same time interval. Therefore, when comparing indices at different time points, the size of the group was smaller than 36. When comparing T0 and T1, the group size was 24, and when comparing T0 and T2, it was 13.(Fig. 2)
Data were statistically analyzed through IBM SPSS Statistics (ver. 25.0; IBM, Armonk, NY, USA). A Shapiro–Wilk test was used to determine the normality of the data. Intraclass correlation coefficient (ICC) and Pearson correlation coefficient were calculated to evaluate intra-rater reliability. Paired t-tests with a significance level of 5% were used to compare the cortical thickness of patients from each group.
The medical records from 36 patients were analyzed. The mean age of the patients was 74.86 years, three of whom were male and the others were female.(Table 1) Mean follow-up length was 20.9 months. Index values of T0, T1, and T2 were compared with paired t-test, because all values showed a normal distribution (Shapiro–Wilk test, P>0.05). Results of comparison are summarized in Fig. 3.
When comparing PMI(I) values at the starting point of drug discontinuation (T0) and 12 months after (T1), mean values were 0.191 and 0.190, respectively. This difference was not statistically significant (P>0.05).
When comparing the starting point of drug discontinuation (T0) and 18-24 months after (T2), the mean values were 0.209 and 0.182, respectively. This difference was statistically significant (P<0.05).(Table 2)
When comparing PMI(S) values at the starting point of drug discontinuation (T0) and 12 months after (T1), the mean values were 0.157 and 0.156, respectively. This difference was not statistically significant (P>0.05).
When comparing the starting point of drug discontinuation (T0) and 18-24 months after (T2), the mean values were 0.173 and 0.152, respectively. This difference was statistically significant (P<0.05).(Table 3)
When comparing MI values at the starting point of drug discontinuation (T0) and 12 months after (T1), mean values were 2.54 and 2.47, respectively. This difference was not statistically significant (P>0.05).
When comparing the starting point of drug discontinuation (T0) and 18-24 months (T2), mean values were 2.77 and 2.42, respectively. This difference was statistically significant (P<0.05).(Table 4)
Intra-rater reliability was evaluated using the ICC and Pearson correlation coefficient. Fifty-nine sets of data pair (1st and 2nd measurement) from randomly selected 9 patients were used. The ICC (3,1) for each variable (I, S, and C) was 0.994, 0.995, and 0.993, respectively. The Pearson correlation coefficient was 0.990, 0.991, and 0.988, respectively.(Table 5)
Horner and Devlin6 found a correlation between bone mineral density, PMI, and MI. Taguchi et al.7 also found a linear correlation between TBMD (total mean bone mineral density) and mandibular inferior cortex height. In Klemetti’s study8, there was a high correlation between patient OST (osteoporosis self-assessment tool) score, PMI, and cortical height. Further, this correlation is still utilized by severa recent studies on bone density. Koh et al.9 used fractal analysis on dental panoramic imaging, showing the fractal dimension value decreasing as bone mineral density decreased. Vlasiadis et al.10 reported a connection between T-score and mandibular cortical width. Kavitha et al.11 used a support-vector machine with cortical thickness on panoramic view to diagnose postmenopausal women with low BMD, which showed good efficacy. In summary, PMI and MI are correlated with bone mineral density and are still in use today. In this way, we inferred changes in bone remodeling rate by changes in mandibular cortical thickness (PMI and MI). To our knowledge, this is the first attempt to observe changes during bisphosphonate drug holiday.
In case of mandibles not affected by bisphosphonate or any other bone-modifying drugs, Roberts et al.12 found a quadratic decrease in mandibular cortical thickness in female individuals after 42.5 years of age and a slow linear decrease in males after 36 years. On the other hand, if the mandible is under bisphosphonate’s effect, Torres et al.13 found that mandibular cortical thickness on cone-beam computed tomography was higher with MRONJ patients compared to control patients. They further reported the same result using dental panoramic radiographs while comparing patients taking bisphosphonate with control patients14. It could be assumed that bisphosphonate increases cortical thickness or at least prevents its decrease, which needs to be confirmed by future longitudinal study.
This study shows that PMI and MI do not decrease until after 12 months of drug holiday (T1). This may be due to bisphosphonate accumulation in the human body. Studies revealed that bisphosphonates bind to free hydroxyapatite, which results in retention in the human body for several years15,16. However, there was a decrease in indices after 18 months of drug discontinuation (T2). Contrary to the estimated half-life of the drug, cortical height change was observed before the actual half-life of the drug.
Drug holiday recommendations for MRONJ patients remain controversial. Ottesen et al.17 concluded that bisphosphonate drug holiday lacked solid evidence on systemic review. Ruggiero et al.3 reported they could not reach a consensus on the use of drug holiday for prevention of MRONJ as the committee was evenly divided on drug holiday efficacy. On the other hand, for established MRONJ patients, it was suggested that drug holiday be considered for those with risk factors such as rheumatoid arthritis, a history of glucocorticoid use, or diabetes mellitus4. An animal study by Zandi et al.18 found that discontinuation of bisphosphonate before and after dentoalveolar surgery contributes to resolution of MRONJ. On the other hand, another animal study found that discontinuation of bisphosphonate did not prevent development of MRONJ in rats. Bisphosphonate’s high affinity to bone was expected to be the cause19. This study also confirmed this effect in that PMI and MI did not show a decrease at 12 months (T1). However, cortical height and PMI showed decrease in 18-24 months (T2). This could indicate osteoclastic activity resumes in 18-24 months. A future study comparing bisphosphonate patients and control patients is needed to confirm this point.
This study could not avoid limitations, including small group size, indirectly quantified bone turnover rate, two-dimensional imaging, and human error in defining the border of mandible. Computed tomography imaging and computer-automated process of measurement would be great additions to future studies.
Thickness change of mandibular cortical bone was evaluated on panoramic radiographs during discontinuation of bisphosphonate (drug holiday). Although there was little change in the first 12 months (T1), the cortical thickness of the mandible decreased after 18 months (T2), which presents radiologic evidence of the skeletal effects of drug holiday.
Notes
Authors’ Contributions
D.H.L. participated in data collection and wrote the manuscript. D.H.L., J.I.S., J.K.L., and S.I.S. participated in the study design. D.H.L. and J.I.S. performed the statistical analysis. J.K.L. and S.I.S. helped to draft the manuscript. All authors read and approved the final manuscript.
References
1. Dayisoylu EH, Şenel FÇ, Üngör C, Tosun E, Çankaya M, Ersöz S, et al. 2013; The effects of adjunctive parathyroid hormone injection on bisphosphonate-related osteonecrosis of the jaws: an animal study. Int J Oral Maxillofac Surg. 42:1475–80. https://doi.org/10.1016/j.ijom.2013.05.001. DOI: 10.1016/j.ijom.2013.05.001. PMID: 23746422.

2. Harper RP, Fung E. 2007; Resolution of bisphosphonate-associated osteonecrosis of the mandible: possible application for intermittent low-dose parathyroid hormone [rhPTH(1-34)]. J Oral Maxillofac Surg. 65:573–80. https://doi.org/10.1016/j.joms.2006.10.076. DOI: 10.1016/j.joms.2006.10.076. PMID: 17307613.

3. Ruggiero SL, Dodson TB, Aghaloo T, Carlson ER, Ward BB, Kademani D. 2022; American Association of Oral and Maxillofacial Surgeonsʼ position paper on medication-related osteonecrosis of the jaws-2022 update. J Oral Maxillofac Surg. 80:920–43. https://doi.org/10.1016/j.joms.2022.02.008. DOI: 10.1016/j.joms.2022.02.008. PMID: 35300956.

4. Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, et al. 2014; ; American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw--2014 update. J Oral Maxillofac Surg. 72:1938–56. https://doi.org/10.1016/j.joms.2014.04.031. DOI: 10.1016/j.joms.2014.04.031. PMID: 25234529.

5. Ledgerton D, Horner K, Devlin H, Worthington H. 1997; Panoramic mandibular index as a radiomorphometric tool: an assessment of precision. Dentomaxillofac Radiol. 26:95–100. https://doi.org/10.1038/sj.dmfr.4600215. DOI: 10.1038/sj.dmfr.4600215. PMID: 9442624.

6. Horner K, Devlin H. 1998; The relationship between mandibular bone mineral density and panoramic radiographic measurements. J Dent. 26:337–43. https://doi.org/10.1016/s0300-5712(97)00020-1. DOI: 10.1016/S0300-5712(97)00020-1. PMID: 9611939.

7. Taguchi A, Suei Y, Ohtsuka M, Otani K, Tanimoto K, Ohtaki M. 1996; Usefulness of panoramic radiography in the diagnosis of postmenopausal osteoporosis in women. Width and morphology of inferior cortex of the mandible. Dentomaxillofac Radiol. 25:263–7. https://doi.org/10.1259/dmfr.25.5.9161180. DOI: 10.1259/dmfr.25.5.9161180. PMID: 9161180.

8. Klemetti E, Kolmakov S, Kröger H. 1994; Pantomography in assessment of the osteoporosis risk group. Scand J Dent Res. 102:68–72. https://doi.org/10.1111/j.1600-0722.1994.tb01156.x. DOI: 10.1111/j.1600-0722.1994.tb01156.x. PMID: 8153584.

9. Koh KJ, Park HN, Kim KA. 2012; Prediction of age-related osteoporosis using fractal analysis on panoramic radiographs. Imaging Sci Dent. 42:231–5. https://doi.org/10.5624/isd.2012.42.4.231. DOI: 10.5624/isd.2012.42.4.231. PMID: 23301209. PMCID: PMC3534177.

10. Vlasiadis KZ, Skouteris CA, Velegrakis GA, Fragouli I, Neratzoulakis JM, Damilakis J, et al. 2007; Mandibular radiomorphometric measurements as indicators of possible osteoporosis in postmenopausal women. Maturitas. 58:226–35. https://doi.org/10.1016/j.maturitas.2007.08.014. DOI: 10.1016/j.maturitas.2007.08.014. PMID: 17923346.

11. Kavitha MS, Asano A, Taguchi A, Kurita T, Sanada M. 2012; Diagnosis of osteoporosis from dental panoramic radiographs using the support vector machine method in a computer-aided system. BMC Med Imaging. 12:1. https://doi.org/10.1186/1471-2342-12-1. DOI: 10.1186/1471-2342-12-1. PMID: 22248480. PMCID: PMC3269982.

12. Roberts M, Yuan J, Graham J, Jacobs R, Devlin H. 2011; Changes in mandibular cortical width measurements with age in men and women. Osteoporos Int. 22:1915–25. https://doi.org/10.1007/s00198-010-1410-3. DOI: 10.1007/s00198-010-1410-3. PMID: 20886206.

13. Torres SR, Chen CS, Leroux BG, Lee PP, Hollender LG, Santos EC, et al. 2012; Mandibular cortical bone evaluation on cone beam computed tomography images of patients with bisphosphonate-related osteonecrosis of the jaw. Oral Surg Oral Med Oral Pathol Oral Radiol. 113:695–703. https://doi.org/10.1016/j.oooo.2011.11.011. DOI: 10.1016/j.oooo.2011.11.011. PMID: 22668629.

14. Torres SR, Chen CS, Leroux BG, Lee PP, Hollender LG, Lloid M, et al. 2015; Mandibular inferior cortical bone thickness on panoramic radiographs in patients using bisphosphonates. Oral Surg Oral Med Oral Pathol Oral Radiol. 119:584–92. https://doi.org/10.1016/j.oooo.2015.02.005. DOI: 10.1016/j.oooo.2015.02.005. PMID: 25864820. PMCID: PMC4395858.

15. Ruggiero SL, Fantasia J, Carlson E. 2006; Bisphosphonate-related osteonecrosis of the jaw: background and guidelines for diagnosis, staging and management. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 102:433–41. https://doi.org/10.1016/j.tripleo.2006.06.004. DOI: 10.1016/j.tripleo.2006.06.004. PMID: 16997108.

16. Cremers SC, Pillai G, Papapoulos SE. 2005; Pharmacokinetics/pharmacodynamics of bisphosphonates: use for optimisation of intermittent therapy for osteoporosis. Clin Pharmacokinet. 44:551–70. https://doi.org/10.2165/00003088-200544060-00001. DOI: 10.2165/00003088-200544060-00001. PMID: 15932344.

17. Ottesen C, Schiodt M, Gotfredsen K. 2020; Efficacy of a high-dose antiresorptive drug holiday to reduce the risk of medication-related osteonecrosis of the jaw (MRONJ): a systematic review. Heliyon. 6:e03795. https://doi.org/10.1016/j.heliyon.2020.e03795. DOI: 10.1016/j.heliyon.2020.e03795. PMID: 32373730. PMCID: PMC7191576.

18. Zandi M, Dehghan A, Ghadermazi K, Malekzadeh H, Akbarzadeh M. 2015; Perioperative discontinuation of intravenous bisphosphonate therapy reduces the incidence and severity of bisphosphonate-related osteonecrosis of the jaw: a randomized, controlled, prospective experimental study in rats. J Craniomaxillofac Surg. 43:1823–8. https://doi.org/10.1016/j.jcms.2015.08.008. DOI: 10.1016/j.jcms.2015.08.008. PMID: 26355024.

19. de Molon RS, Shimamoto H, Bezouglaia O, Pirih FQ, Dry SM, Kostenuik P, et al. 2015; OPG-Fc but not zoledronic acid discontinuation reverses osteonecrosis of the jaws (ONJ) in mice. J Bone Miner Res. 30:1627–40. https://doi.org/10.1002/jbmr.2490. DOI: 10.1002/jbmr.2490. PMID: 25727550. PMCID: PMC4995600.

Fig. 1
Defining landmarks by Ledgerton’s method. Panoramic mandibular index (PMI) and mental index (MI), S=the distance from the superior border of mental foramen to mandibular border, I=the distance from the inferior border to mandibular border, C=cortical bone thickness, that is MI.
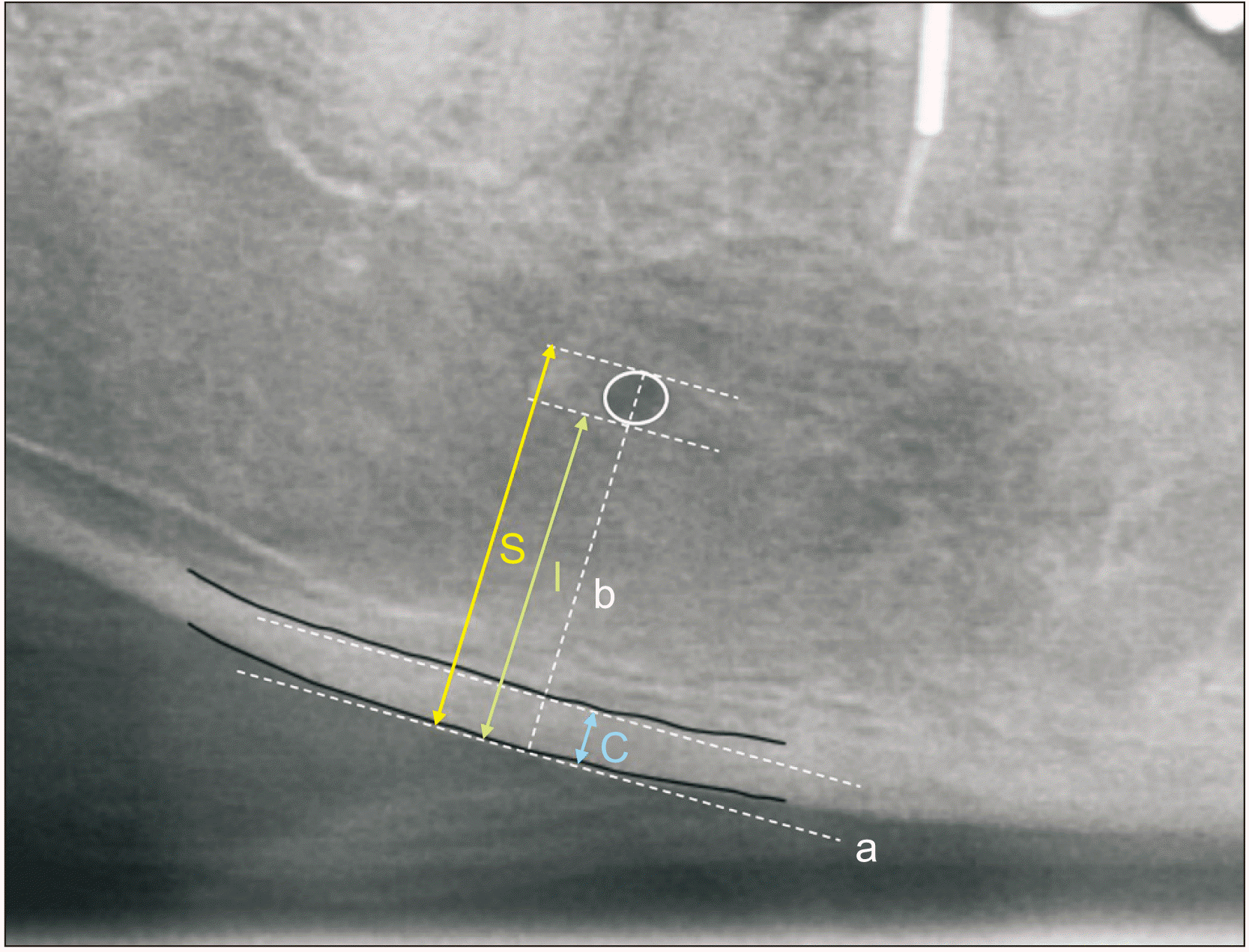
Fig. 2
Groups of patients according to drug holiday length. When comparing T0 and T1, the size of group was 24, patients of which have taken dental panoramic radiograph at starting point of drug discontinuation and 12 months from the start. When comparing T0 and T2, the size of group was 13, patients of which have taken the radiograph at starting point of drug discontinuation and 18-24 months from the start.
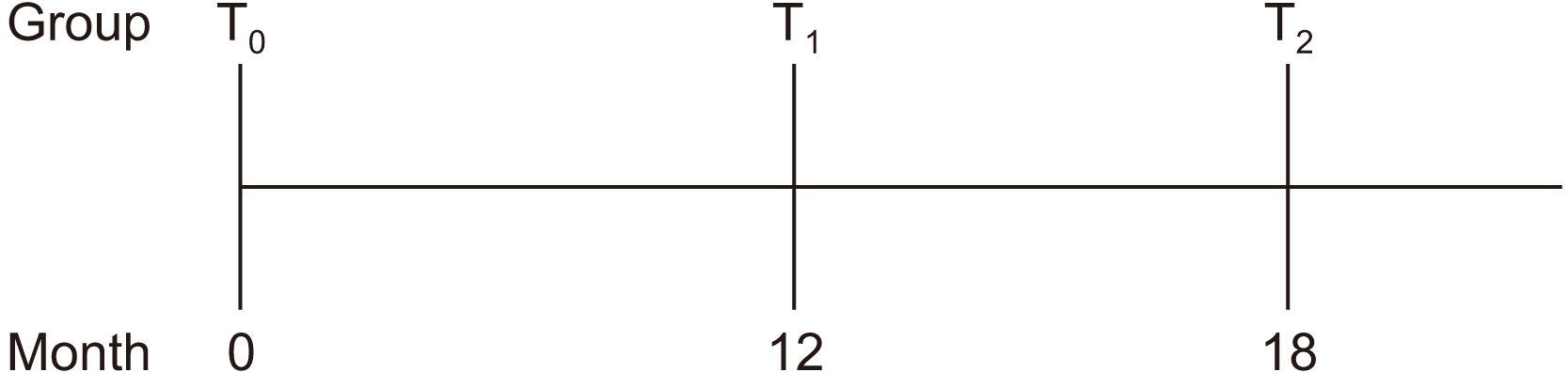
Fig. 3
Comparison of index values. MI=mental index, PMI(I and S)=panoramic mandibular index. When comparing T0 and T1, the size of the group was 24. When comparing T1 and T2, the size of the group was 13.
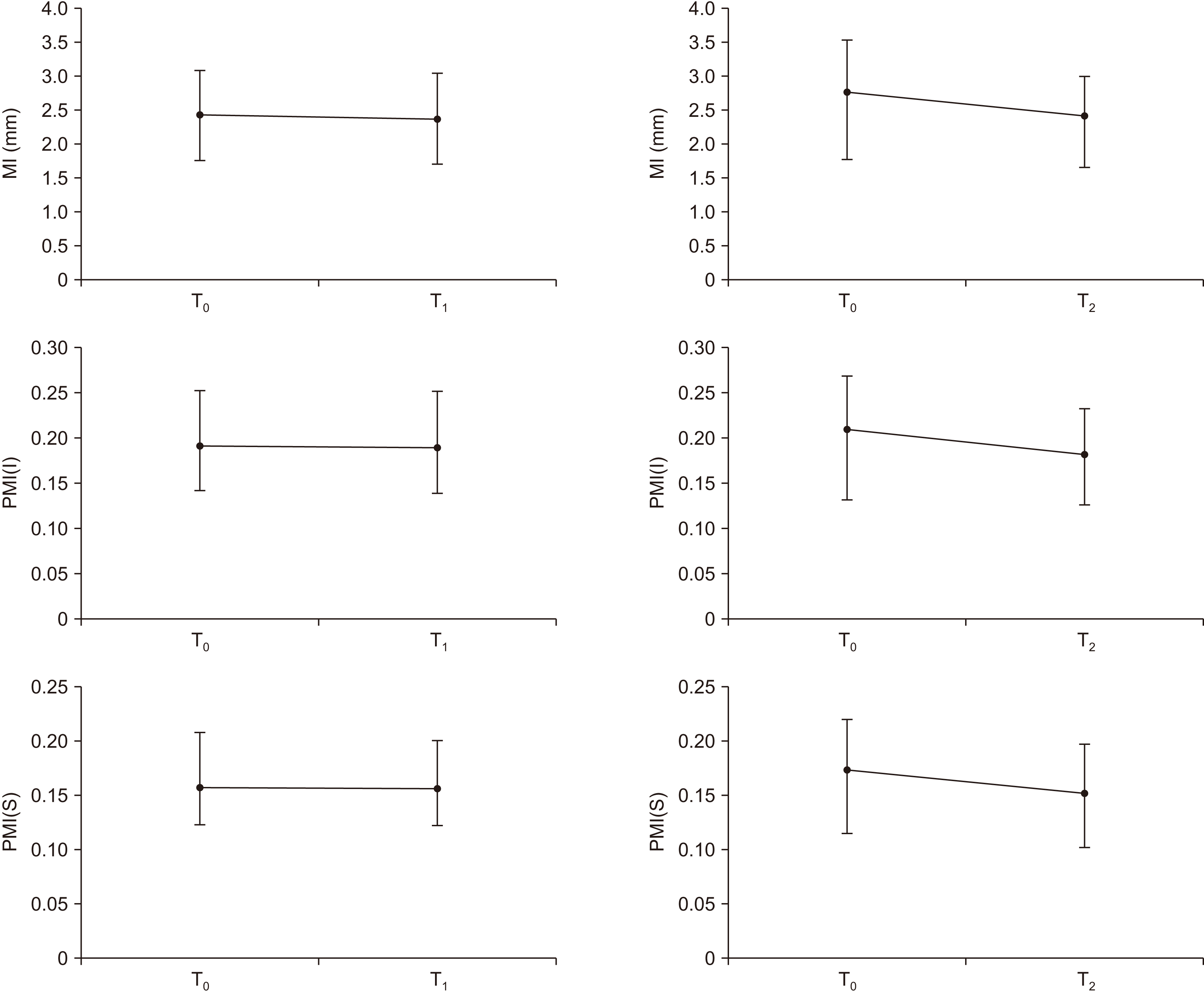
Table 1
Patient demographics
Table 2
Mean measurement values and P-value of comparison, PMI(I)
| Group size | T0 | T1 | T2 | P-value |
|---|---|---|---|---|
| n=24 | 0.191 | 0.190 | - | 0.683 |
| n=13 | 0.209 | - | 0.182 | 0.007 |
Table 3
Mean measurement values and P-value of comparison, PMI(S)
| Group size | T0 | T1 | T2 | P-value |
|---|---|---|---|---|
| n=24 | 0.157 | 0.156 | - | 0.740 |
| n=13 | 0.173 | - | 0.152 | 0.006 |
Table 4
Mean measurement values and P-value of comparison, MI (unit: mm)
| Group size | T0 | T1 | T2 | P-value |
|---|---|---|---|---|
| n=24 | 2.54 | 2.47 | - | 0.299 |
| n=13 | 2.77 | - | 2.42 | 0.003 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download