This article has been
cited by other articles in ScienceCentral.
Abstract
Objectives
This study aims to evaluate the efficacy and safety of two types of sandblasted with large-grit and acid-etched (SLA) surface implants with different surface roughness.
Patients and Methods This study was conducted based on a clinical record review of 55 patients (mean age, 53.00 years). A total of 80 SLA surface implants was placed. Among the 80 implants, 38 implants placed in 29 subjects had surface roughness (Ra) of 3.09 µm (test group, TG), while the other 42 implants placed in 31 subjects had a surface roughness (Ra) of 2.50 µm (control group, CG). A comparison was made of implant primary/secondary stability; success and survival rates; marginal bone loss; and soft tissue assessment including probing pocket depth (PPD), plaque index (PI), gingival index (GI), and bleeding on probing (BOP) between the groups at 1 year after implant placement.
Results
Among the implants that were initially registered, 1 from the TG and 4 from the CG dropped out, leaving 37 implants in the TG and 38 implants in the CG to be traced and analyzed. Although 1 TG case showed unstable primary stability, all cases showed stable secondary stability. Success and survival rates at 1 year after implant placement were 100% in both groups. Marginal bone loss was 0.07 mm and 0.00 mm for the TG and CG, respectively, but the difference was not significant. Among the several parameters for evaluation of soft tissue, the TG showed lower PI at 1 year after implant placement (TG=0.00, CG=0.29; P=0.0004), while the remaining categories showed no significant difference between the groups.
Conclusion
This study shows that the two types of SLA implants with different surface roughness have no difference in efficacy or safety. Therefore, both of the implants can be used safely and with promising outcomes.
Go to :

Keywords: Dental implants, Osseointegration, SLA, Surface roughness
I. Introduction
The stability of a dental implant depends on the level of osseointegration, which is affected by several factors such as the shape, structure, and surface of the implant. Early titanium implants had a smooth, mechanically polished surface, but studies revealed that implants with a rough surface are more stable and superior in the long term
1,2. The purpose of surface treatment of implants is to improve osseointegration between the bone and the implant by generating micro-irregularities that produce a larger contact area
3. Accordingly, various methods have been proposed to increase the initial fixation force based on mechanical and chemical stimulation of the implant surface. Examples of such treatments include a blasting method that sprays TiO
2 or Al
2O
3 particles to scratch the surface, an acid-etched method that erodes the implant surface with a high-temperature acidic solution to increase the roughness, a porous sintering method, an anodizing method, and sandblasted with large-grit and acid-etched (SLA) methods
4,5.
Recently, the SLA method, which combines the blasting and acid-etch methods, has been widely applied in implant processing. The SLA method increases surface roughness (Ra) at both the macro and micro levels because acid corrosion after particle blasting further disrupts the surface. This method fosters increased osseointegration by facilitating contact of the rough surface with a larger number of osteoblasts and their surface energy compared with a smooth surface. The reported 10-year survival rate and incidence of peri-implantitis of SLA surface implants are 99.7% and 7.0%, respectively, indicating an adequate long-term prognosis
6,7. Goyal and Kaur
8 observed that increased roughness can simultaneously increase the surface area of the implant, improve cell migration and attachment to the implant, and enhance the osseointegration process. However, excessively increasing Ra without consideration of the characteristics of the implant can prevent bone from adhering to the implant surface. Therefore, the optimal Ra of the implant should be dependent upon the characteristics of the surface treatements done on the implants
9.
According to Knabe et al.
10, for SLA implants, it is suggested that an Ra of 3.43 µm exhibits the highest stability. The Ra of one of the most widely used SLA implants approved in Korea is 2.50 µm. Therefore, Sewon Medics has developed an SLA implant with further increased Ra while maintaining other conditions. This clinical study was conducted to evaluate the safety and effectiveness of SLA implants with increased Ra compared to previously-licensed SLA implants.
Go to :

II. Patients and Methods
This prospective clinical study was conducted after receiving approval from the Institutional Review Board (IRB) of Seoul National University Bundang Hospital (IRB No. E-1909-564-003). From March 2020 to September 2021, 55 subjects who underwent dental implant placement due to tooth loss at Seoul National University Bundang Hospital Dental Clinic were registered. Two types of implants with different Ra were used, both of which were SLA surface, internal connection, titanium implants. The implant used in the test group (TG) was IH2 SLA fixture (Sewon Medics, Busan, Korea), with an Ra of 3.09 µm. The implant used in the control group (CG) was TSIII SA (Osstem Implant, Seoul, Korea), which has an Ra of 2.50 µm and is currently licensed and widely used in Korea.
In this clinical trial, 55 patients were enrolled according to strict pre-defined inclusion and exclusion criteria.(
Table 1) A total of 80 SLA implants were randomly assigned to the TG and CG and placed in 55 healthy subjects who provided written informed consent to implant surgery for restoration of missing teeth. Of these, 38 implants in 29 subjects were included in the TG and 42 implants in 31 subjects were included in the CG. Five subjects received both TG and CG implants.
Table 1
Inclusion and exclusion criteria
|
Inclusion criteria |
Age over 19 years |
|
Patients with at least one (maximum 3) missing teeth needing implantation |
|
Patients with fair oral hygiene |
|
For fertile female patients who have agreed on contraception |
|
Patients who voluntarily participated in the research |
|
Patients willing to abide by the program |
|
Predicted implant site has 1-3 quality bone density and a sufficient quantity of bone |
|
Opposing teeth and adjacent teeth exist and will not undergo implantation within the follow-up period |
|
Exclusion criteria |
Patients with severe periodontitis or infection |
|
Patients with bone lesions or a relevant surgical procedure |
|
Patients with bone-related disease |
|
Patients who smoke 20 or more cigarettes per day |
|
Alcohol or drug addiction |
|
Patients with severe clenching or bruxism |
|
Patients who underwent extraction <8 weeks before |
|
Patients who had radiation therapy within 6 months |
|
Patients who received any kind of GBR (guided bone regeneration) within 6 months |
|
Systemic condition that contraindicates oral surgical procedures |
|
Pregnant or lactating or the possibility of becoming pregnant |
|
Cases that need bone augmentation |

All implants were placed by a single surgeon without bone graft. Immediately after implant placement, primary stability (implant stability quotient, ISQ) was measured using an Osstell Mentor (Osstell, Gothenburg, Sweden). The second surgery was performed 20 weeks after implant placement in the upper jaw and 8 weeks after implant placement in the mandible. Secondary stability was measured during the second surgery using the Osstell Mentor. The threshold of implant stability measured by ISQ was set to 60 based on the results of Rodrigo et al.
11, where it was reported that implant failure rarely occurs when ISQ >60 and failure rates rise to 19% when ISQ is >60
11,12. The final implant prosthesis was loaded with a single crown by a single prosthodontist 4 weeks after the second surgery.
Medical records and radiographic information (panoramic view, periapical view) were used to evaluate implant success and survival rates, marginal bone loss, and soft tissue factors including probing pocket depth (PPD), plaque index (PI), gingival index (GI), and bleeding on probing (BOP) at 1 year after implant placement.
1. Success rate
The implant success rate 1 year after placement was compared between groups as follows.
Success of the implant was noted when all of the following conditions were satisfied
13: 1) The implant was not fractured and survived in the placed position; 2) There was no persistent pain, foreign body sensation, or dysesthesia; 3) There was no inflammation accompanied by suppuration around the implant; 4) The PPD on the buccal (or labial), palatal (or lingual), mesial, and distal sides was less than 5 mm; 5) The implant showed no mobility; and 6) There was no radiolucent lesion around the implant and no marginal bone loss on apical radiographic examination.
2. Survival rate
The implant survival rate at 1 year after placement was compared and evaluated between the groups as follows.
Implant survival was defined as implant persistence at the implanted site.
3. Marginal bone loss
The amount of marginal bone loss at 1 year after implant placement was evaluated using a baseline periapical radiograph collected 2 weeks after implant placement. Marginal bone loss was measured as the distance between the shoulder of the implant and the uppermost part of the bone in contact with the implant and averaged between the mesial and distal sides. The measurements were calculated as a proportion of the actual length of the implant and the length on radiographs.(
Fig. 1)
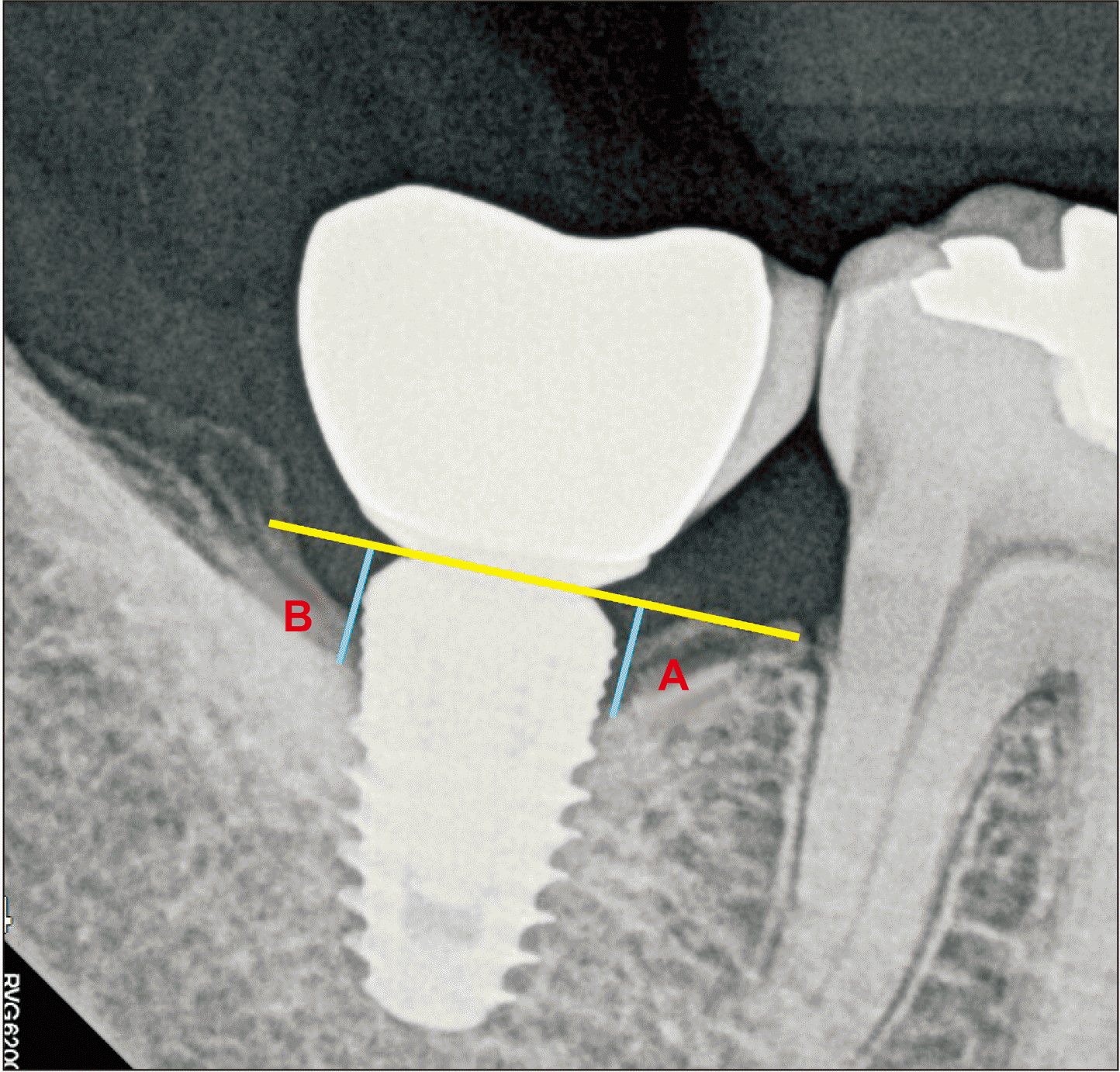 | Fig. 1Landmarks of the radiographic measurements. A point: Linear distance from implant shoulder to contact point of implant and bone (mesial surface), B point: Linear distance from implant shoulder to contact point of implant and bone (distal surface). The mean value of A point and B point was set as the marginal bone resorption amount. 
|
4. Soft tissue evaluation
Soft tissue evaluation involved examination of PPD, PI, GI, and BOP. PPD was measured using a periodontal probe at four sites (buccal [or labial], palatal [or lingual], mesial, and distal) along the tooth
14. PI was evaluated as follows: 0 for no plaque, 1 for a thin plaque layer at the gingival margin, only detectable by scraping with a probe, 2 for a moderate layer of plaque along the gingival margin, interdental spaces, and free but when plaque is visible to the naked eye, and 3 for abundant plaque along the gingival margin, interdental spaces filled with plaque. The Lebene GI was evaluated as follows: 0 for normal gingiva, no inflammation, no discoloration, no bleeding, 1 for mild inflammation, slight erythema, minimal superficial alterations, no bleeding, 2 for moderate inflammation, erythema, BOP, and 3 for severe inflammation, severe erythema and swelling, spontaneous bleeding and possible ulceration. BOP was evaluated positive if bleeding occurred within 30 seconds after periodontal probing and negative if not
14.
The results of primary and secondary stability, survival rate, success rate, marginal bone loss, and soft tissue evaluation were analyzed using IBM SPSS Statistics (ver. 25.0; IBM, Armonk, NY, USA). Statistical analysis was performed with the chi-square test, Fisher’s exact test, and multiple regression analysis, which tested the significance of any association at a level of 95%.
Go to :

III. Results
Among the 38 TG and 42 CG implants that were initially registered, 1 TG patient and 2 CG patients dropped out during follow-up. The TG patient failed to complete follow-up, and the 2 CG patients had more than 3 missing teeth 8 weeks before implantation and thus did not meet the inclusion category. Therefore, 37 TG implants and 38 CG implants were followed and analyzed in this clinical trial, as shown in
Table 2. Analysis of basic demographic data (sex, age, severe clenching/bruxism, and drinking and smoking habits) was conducted, and no statistically significant differences were found between the two groups.(
Table 3)
Table 2
Number of implants and subjects by group
|
Group |
Registered |
Lost |
Final |
|
Test group |
38 (29) |
1 (1) |
37 (28) |
|
Control group |
42 (31) |
4 (2) |
38 (29) |
|
Total |
80 (55)1
|
5 (3) |
75 (52) |

Table 3
Demographic analysis between groups
|
TG |
CG |
P-value |
|
Sex |
|
|
0.8930 |
|
Male |
16 |
16 |
|
|
Female |
12 |
13 |
|
|
Age (yr) |
|
|
0.1387 |
|
Number |
28 |
29 |
|
|
Mean±SD |
55.10±10.54 |
50.83±11.64 |
|
|
Median |
58.00 |
54.00 |
|
|
Min, Max |
27.00, 73.00 |
23.00, 71.00 |
|
|
Severe clenching/bruxism |
|
|
- |
|
Yes |
0 |
0 |
|
|
No |
28 |
29 |
|
|
Drinking |
|
|
0.7072 |
|
Yes |
3 |
4 |
|
|
No |
25 |
25 |
|
|
Smoking |
|
|
0.6713 |
|
Yes |
2 |
5 |
|
|
No |
26 |
24 |
|

The implantation sites were 4 maxillary premolars, 5 maxillary molars, 5 mandibular premolars, and 23 mandibular molars in the TG and 2 maxillary incisors and 3 maxillary premolars, 5 maxillary molars, 5 mandibular premolars, and 23 mandibular molars in the CG, as shown in
Table 4.
Table 4
Implantation site by group (unit: No. of implants)
|
Group |
Anterior |
Premolar |
Molar |
Total (%) |
|
TG (n=37) |
|
|
|
|
|
Upper jaw |
- |
4 |
5 |
9 (24.3) |
|
Lower jaw |
- |
5 |
23 |
28 (75.7) |
|
CG (n=38) |
|
|
|
|
|
Upper jaw |
2 |
3 |
5 |
10 (26.3) |
|
Lower jaw |
- |
5 |
23 |
28 (73.7) |

The mean primary stability of implants in the 37 cases in the TG was 79.7 ISQ, and a single case showed a primary stability lower than 60. The mean primary stability of the 38 cases in the CG was 82.9 ISQ, and none of the cases showed stability lower than 60. Also, mean secondary stability was 82.1 ISQ in the TG and 84.4 ISQ in the CG, and none of the cases showed values lower than 60.(
Table 5)
Table 5
Implant primary/secondary stability
|
Group |
Primary stability (ISQ) |
|
Secondary stability (ISQ) |
|
|
|
Mean±SD |
<60 |
Mean±SD |
<60 |
|
TG (n=37) |
79.7±10.22 |
1 |
82.1±11.12 |
0 |
|
CG (n=38) |
82.9±10.00 |
0 |
84.4±10.31 |
0 |
|
P-value |
>0.999 |
|
>0.999 |
|

The success and survival rates 1 year after implant placement were 100% for both groups. Marginal bone loss 1 year after implant placement was 0.07 mm for the TG and 0.00 mm for the CG, but this difference was not significant (
P=0.0778).(
Table 6)
Table 6
Evaluation of the success rate, survival rate, and marginal bone loss (MBL) 1 year after implant placement
|
Group |
Success rate (%) |
Survival rate (%) |
MBL (mm) |
|
TG (n=37) |
100 |
100 |
0.07 |
|
CG (n=38) |
100 |
100 |
0.00 |
|
P-value |
- |
- |
0.0778 |

The evaluation of soft tissue 1 year after implant placement showed a significantly lower PI in the TG compared to the CG (TG=0.00, CG=0.29;
P=0.0004), while the other categories were not significantly different between groups.(
Tables 7,
8)
Table 7
Evaluation of soft tissue 1 year after implant placement (categorical analysis)
|
TG (n=37) |
CG (n=38) |
Total (n=75) |
P-value |
|
PPD (mm) |
3.01 |
2.95 |
2.98 |
0.2690 |
|
PI |
|
|
|
0.0004* |
|
0 |
37 |
27 |
64 |
|
|
1 |
0 |
11 |
11 |
|
|
2 |
0 |
0 |
0 |
|
|
3 |
0 |
0 |
0 |
|
|
GI |
32 |
32 |
64 |
0.7806 |
|
0 |
5 |
6 |
11 |
|
|
1 |
0 |
0 |
0 |
|
|
2 |
0 |
0 |
0 |
|
|
3 |
|
|
|
|
|
BOP |
|
|
|
- |
|
Yes |
0 |
0 |
0 |
|
|
No |
37 |
38 |
75 |
|

Table 8
Evaluation of soft tissue 1 year after implant placement (continuous analysis)
|
TG (n=37) |
CG (n=38) |
Total (n=75) |
P-value |
|
PPD (mm) |
|
|
|
0.2690 |
|
Number |
37 |
38 |
75 |
|
|
Mean |
3.01 |
2.95 |
2.98 |
|
|
Median |
3.00 |
3.00 |
3.00 |
|
|
Min, Max |
2.50, 3.75 |
2.00, 4.00 |
2.00, 4.00 |
|
|
PI |
|
|
|
0.0004* |
|
Number |
37 |
38 |
75 |
|
|
Mean |
0.00 |
0.29 |
0.15 |
|
|
Median |
0.00 |
0.00 |
0.00 |
|
|
Min, Max |
0.00, 0.00 |
0.00, 1.00 |
0.00, 1.00 |
|
|
GI |
|
|
|
0.7887 |
|
Number |
37 |
38 |
75 |
|
|
Mean |
0.14 |
0.16 |
0.15 |
|
|
Median |
0.00 |
0.00 |
0.00 |
|
|
Min, Max |
0.00, 1.00 |
0.00, 1.00 |
0.00, 1.00 |
|

Go to :

IV. Discussion
Dental implants have become a universal treatment method for restoration of lost teeth. Many advancements in implant shape and surface have been made in order to promote osseointegration and ensure long-term stability
15. Dental implants started out as a machined, smooth-surface external-type implants, now known as the Branemark system, after which internal connection-type implants were adopted, which are now more widely used
16-18. Different types of implant fixtures have been introduced for different purposes. A straight body allows easy control of implantation depth and has an excellent self-tapping ability, while a tapered body is useful for immediate implantation after tooth extraction and shows excellent initial fixation by compacting trabecular bone when needed
19-21. However, according to Jang et al.
22, there is no significant difference in long-term clinical prognosis between tapered and straight implants, and the selection should be based on the implantation site and operator’s preference. Implant surface treatment improves the wettability of the surface and increases bone-implant contact to improve osseointegration. The SLA method, which currently is the most widely used technique, employs TiO
2 or Al
2O
3 particles for surface abrasion, and several studies mention that 75-µm aluminum particles are most effective for sandblasting. The second step, acid treatment, increases micro-roughness to further increase osseointegration
23-26.
This study was conducted to compare the short-term clinical prognosis of two internal connection-type, tapered, SLA-surface implants with different surface roughness. The implant used for the TG was the submerged-type IH2 SLA Fixture developed by Sewon Medics and is a titanium internal hex with an 11-degree Morse taper structure. Surface roughness with this implant was 3.09 µm after SLA treatment. The implant used for the CG was the common TSIII SA implant developed by Osstem Implant, which is also a submerged-type, titanium internal hex with an 11-degree Morse taper structure. The surface roughness of the TSIII SA is 2.50 µm.
In this clinical trial, complications were reported in 3 cases (TG: 3 cases, CG: 0 case). The 3 treatment site complications were ‘implant site discharge,’ ‘implant site swelling,’ and ‘implant site hemorrhage,’ all of which are classified as ‘General disorders and administration site conditions’ according to MedDRA’s SOC (System Organ Class) criteria. All 3 complications were ‘mild’ and had little or no relevance to the implant procedure. All 3 cases recovered before final follow-up. Both types of SLA surface implants compared in this clinical study had a 100% success rate, and marginal bone loss of less than 0.1 mm. Soft tissue measures 1 year after implant placement also showed stable results in both groups. Although 1 case from the TG showed unstable primary stability, all cases eventually showed stable ISQ in terms of secondary stability, proving the effectiveness and safety of both implants.
The implants were placed in patients with relatively good condition, and the second surgery and prosthetic loading proceeded after sufficient healing, producing good clinical results. There was no difference in clinical performance according to surface roughness under the SLA modification. This prospective randomized clinical trial has value as a reference for clinical research for approval of dental devices.
Surface roughness is indisputably an important factor determining dental implant osseointegration. Although the results of this study indicate that an implant with increased surface roughness show similar stability to preexisting implants, the ideal surface roughness for osseointegration and primary stability remains unclear
9. Further innovative studies at the nanoscale level may provide valuable insight into dental implantology.
Go to :

V. Conclusion
This study demonstrated that two types of SLA implants with different surface roughness showed similar efficacy and safety. Both of these implants can be used safely and with promising outcomes.
Go to :






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download