Abstract
Backgrounds/Aims
Thoracic epidural analgesia (TEA) is an established analgesic method in open Kausch-Whipple pancreaticoduodenectomy (KWPD). Although, it can cause hemodynamic instability and neurological complications. Inter pleural analgesia (IPA) is an alternative option. We aim to evaluate the effectiveness of IPA versus TEA after KWPD.
Methods
We retrospectively studied the efficacy of IPA against TEA in patients, operated by a single surgeon. The primary outcome was the analgesic efficacy and secondary outcomes were analgesia-related complications, inotrope use, and duration.
Results
Forty patients (TEA, 22; IPA, 18) were included. Both groups were well matched for patient characteristics, type, and duration of surgery. TEA was associated with higher analgesia-related complications (n = 8, 36.4% vs. n = 1, 5.6%; p = 0.027). TEA complications included analgesia not working (n = 4), leakage (n = 2), refractory hemodynamic instability (n = 1), and lower limb anaesthesia (n = 1). One patient in the IPA group encountered leakage. TEA was associated with longer inotrope requirement (35 vs. 18 hours; p = 0.047). There was no significant difference in intensive care unit (ITU) admission rate (81.8% vs. 77.8%; p > 0.999), median ITU stay (3 vs. 2 days, p = 0.385), or hospital stay (11 days in both groups).
Conclusions
In open KWPD, IPA is not inferior to TEA in its efficacy of pain control. IPA was associated with less analgesia-related complications and shorter inotrope requirements. However, this was a small retrospective study. Larger randomized controlled trials are needed to study the effectiveness of IPA.
Effective management of postoperative pain is crucial in optimizing clinical care following a major upper abdominal surgery. Adequate postoperative pain relief can reduce postoperative complications (particularly respiratory-related complications) and help with enhanced early recovery [1]. Open Kausch-Whipple pancreaticoduodenectomy (KWPD) is a common pancreatic procedure used to treat pancreatic head and peri-ampullary tumors. Traditionally, thoracic epidural analgesia (TEA) is the preferred analgesic option for KWPD and other upper gastrointestinal and thoracic surgeries. Although TEA is an effective analgesic method of choice, it carries a potential risk of complications, including dural perforation, hypotension, bradycardia, and neurological injury [2]. Moreover, the occurrence of systemic hypotension may require additional inotropic support [3] and extended intensive care unit (ITU) admission, resulting in further related complications and higher resource demands. Consequently, an alternative effective analgesic method not associated with these side effects would be preferable over TEA.
Inter pleural analgesia (IPA) is one of such alternative analgesic options that is increasingly used in breast, thoracotomy, and minimally invasive cardiothoracic surgery [4,5]. The technique of IPA was first described in 1993 by Murphy [6]. Analgesia is hypothesized to occur by diffusion of local anesthetic into the parietal pleura, intercostal nerves, and intrathoracic sympathetic chain, thus providing adequate analgesia for unilateral thoracic and upper abdominal pain. IPA catheters (IPAC) enable a continuous administration of local anesthetic agents during the perioperative period. Unlike TEA, IPA does not cause adverse hemodynamic effects. However, it can cause pneumothoraces, although such cases are rare [7,8]. The role of IPA in patients undergoing open KWPD has not been established yet. Thus, the aim of this study was to evaluate the effectiveness of IPA compared to TEA in open KWPD.
This retrospective comparative study reviewed all patients who underwent KWPD by a single surgeon (SA) at University Hospitals Plymouth (UHP) NHS Trust between February 1st, 2013 and June 30th, 2016. The KWPD was carried out using a right-sided ‘reverse L’ incision which consisted of a midline incision that began just below the xiphoid process, extending along the linea alba down to a point above the umbilicus and continuing laterally in a right transverse incision. Patients who had TEA or IPA along with patient-controlled analgesia (PCA) were included in this study. Patients who had alternative analgesia methods such as transabdominal wound catheters (TAWC) or spinal analgesia plus PCA were excluded from this study. The choice of postoperative analgesia was decided solely by the consultant anaesthetist rather than the surgeon depending on whether they had the experience of inserting IPA. Anaesthetists without experience of inserting IPA chose to use TEA and other forms of analgesia over IPA.
We hypothesised that IPA would be similar in its analgesic efficacy compared to TEA. The primary outcome was the analgesic effectiveness of IPA compared to TEA. Pain scores were recorded routinely for patient care rather than specifically for this study. Data were collected by the nursing staff or the acute care team by asking patients to pick from four options: none, mild, moderate, or severe pain. Although the actual pain level might differ between patients due to pain tolerance and individual differences, subjective self-assessment of pain severity nevertheless could provide an idea whether the pain experienced was acceptable to the patient. Pain severity was further categorised as adequate or inadequate pain control. Adequate control was defined as none or mild pain. Inadequate control was defined as moderate or severe pain. Pain severity for intubated patients was not assessed as communication was not possible.
Secondary outcomes included TEA and IPA procedure-related complications, the extent of organ and inotropic support, and intensive unit stay (ITUS). For this study, refractory haemodynamic instability was defined as a cardiovascular compromise (bradycardia and hypotension) unresponsive to simple, non-invasive measures such as fluid resuscitation.
Our unit’s practice is to send patients to intensive care unit/high dependency unit (ITU/HDU) or level 1 facility depending on their performance status and cardiopulmonary exercise testing results following 2 to 4 hours stay in the recovery unit. The unit has a well-established enhanced recovery after surgery (ERAS) pathway, on which all patients are enrolled. The local audit department approved this study.
Patient details were obtained from a prospectively maintained hepato-pancreaticobiliary unit database and electronic patient records. The following data were collected retrospectively from medical notes: patient demographics, mode of analgesia, daily pain assessment scores from postoperative day (POD) zero to five, use of inotropic medication, length of ITUS and total hospital stay.
Inter pleural analgesia
The IPAC was inserted with the patient anesthetized before the surgery, allowing continuous infusion of local anesthetic during the surgery. This could maximize the spread and absorption of analgesia, thus reducing opiate requirements. The IPAC was inserted under sterile conditions, having completed the Prep-Stop-Block process [9]. The patient was positioned supine with their right arms held above their heads. A continuous flow of saline was attached to a 16-G Tuohy needle. Ventilation was turned off for the procedure with the patient at the end of the expiratory phase. The needle was inserted in the mid-axillary line in the thoracic safe triangle (area of the chest wall-bounded anteriorly by pectoralis major, posteriorly by latissimus dorsi, inferiorly by fifth intercostal space and superiorly by the axilla). The needle was inserted onto the body of the rib and walked off the top of the rib, avoiding the intercostal neurovascular bundle. Once the needle tip entered the interpleural space, the negative pressure caused a continuous flow of saline, preventing air entrainment. The catheter was then inserted, leaving approximately 15 cm in the interpleural space. Local anesthetic was administered after securing the catheter. Assessment for pneumothorax was completed using real-time ultrasound or chest X-ray performed after surgery.
Thoracic epidural analgesia technique
Epidural analgesia catheters were inserted according to a well-described technique [10].
The chi-squared test with Yates’ continuity correction was used to compare pain scores between TEA and IPA groups when the frequency of all outcomes was greater than 5. Otherwise, Fisher’s exact test was used. Two-proportions Z-test was used to determine statistical inferiority. The Mann–Whitney U test was used for categorical and continuous outcome variables. Statistical significance was considered when p-value was less than or equal to 0.05. All statistical analyses were performed using R Studio (Version 1.4.1106; R Foundation for Statistical Computing, Vienna, Austria).
A total of 40 patients with a median age of 67 years (interquartile range [IQR], 61–72 years) were included in this study. Twenty-two (55.0%) patients received TEA and 18 (45.0%) patients received IPA. Both groups were well-matched. There were no significant differences in baseline patient characteristics, Charlson Comorbidity Index (CCI), type of surgery, or duration of surgery between the two groups. Further details of patients’ demographics, comorbidities, and operative details of both groups are described in Table 1, 2. Just under half of patients in each group were female: 10 (45.5%) in the TEA group and eight (44.4%) in the IPA group. The sex distribution was not significantly (p = 0.949) different between the two groups. The median age was 68 years (IQR, 62–70 years) in the TEA group and 67 years (IQR, 59–76 years) in the IPA group, showing no significant (p = 0.586) difference between the two. The median ASA score, CCI, and operative duration for both groups were also similar: three (p = 0.925), five (p = 0.888), and six hours (p = 0.854), respectively.
One patient in the TEA group and four patients in the IPA group had preoperative pancreatitis (p = 0.155). The number of additional intra-operative procedures including exploratory laparoscopy, portal vein resection, frozen section, non-regional lymph node resection, and other resections were similar between the two groups (Table 1). No patients required intraoperative blood transfusion.
Median Clavien-Dindo gradings for postoperative complications were 1 (IQR, 0–3) and 2 (IQR, 0–4) for TEA and IPA groups, respectively (Table 1). Three patients in the TEA group and two patients in the IPA group suffered postoperative bleeding. The median duration of ITUS and total hospital stay durations were also comparable between the both analgesia groups.
Daily pain assessment scores are shown in Table 2. Six recordings (TEA, n = 3; IPA, n = 3) were not obtained due to intubation at the time of assessment (POD 0–2). Thirteen (59.1%) patients in the TEA group and seven (38.1%) patients in the IPA group had adequate pain control (p = 0.340) in recovery (POD 0) and during all five PODs (POD 1–5). The remaining nine (40.9%) patients in the TEA group and 11 (61.1%) patients in the IPA group reported moderate or severe pain on at least one occasion.
The median day of TEA or IPA removal was POD 3 for both groups. Thus, POD 0–3 was evaluated in more detail. During this period, there were a total of 154 recorded daily pain scores (Table 3). Of these, there were 59 records of no pain (TEA, n = 32/85 [37.6%]; IPA, n = 27/69 [39.1%]; p = 0.983), 67 records of mild pain (TEA, n = 42/85 [49.4%]; IPA, n = 25/69 [36.2%]; p = 0.140), 25 records of moderate pain (TEA, n = 10/85 [11.8%]; IPA, n = 15/69 [21.7%]; p = 0.147), and three records of severe pain (TEA, n = 1/85 [1.2%]; IPA, n = 2/69 [2.9%]; p = 0.587).
The reported pain severity between POD 0–3 did not show statistically significant difference between the two analgesia methods (p = 0.197). Moderate pain was reported slightly higher by patients in the IPA group (21.7% vs. 11.8%, p = 0.147), but not statistically significant. Further analysis of inferiority, the null hypothesis ‘observed proportion of inadequate pain is greater in the IPA group than the TEA group’ was rejected (p = 0.048).
Analgesic complications encountered were analgesia leakage, analgesia not working, refractory hemodynamic instability and lower limb anesthesia. For this study, ‘analgesia leakage’ was considered when TEA/IPA infusion pumps showed errors either due to fluid leakage from catheters or the entry site of the catheter. ‘Analgesia not working’ was considered when either analgesic infusion was not possible due to a kink inside the epidural space or inter pleural space or when it was not practical or could not be used due to fluid leakage. ‘Refractory hemodynamic instability’ was considered when cardiovascular compromise (bradycardia and hypotension) was unresponsive to simple and non-invasive measures such as fluid resuscitation (Table 4).
Rates of analgesic procedure-related complications were significantly higher with TEA (n = 8, 36.4%) than with IPA (n = 1, 5.6%) (p = 0.027). TEA complications included analgesia not working (n = 4), leakage (n = 2), refractory hemodynamic instability (n = 1), and lower limb anesthesia (n = 1). The only IPA complication encountered was leakage, which was removed early. Seven epidural catheters (leakage = 2, not working = 4, refractory hemodynamic instability = 1) were removed early between POD 0–2. Patients who reported severe pain or whose parental analgesia was removed early were supplemented with additional intravenous morphine.
Respiratory complications rates were comparable between both analgesia methods (TEA = 31.8%; IPA = 33.3%; p > 0.999). There were no incidences of pleural effusion or pneumothorax with IPA or TEA.
Eleven (50.0%) patients in the TEA group and eight (44.4%) patients in the IPA group required inotropic support (p = 0.975). The median duration of inotrope use was significantly longer in the TEA group than in the IPA group; median duration of 35 hours (IQR, 22–59 hours) versus 18 hours (IQR, 2–38 hours) (p = 0.047). We also noted that two patients in the IPA group (CCI: 5 and 7; operative time: 5 and 5.5 hours) only required inotropic support for less than two hours, suggesting a transient compromise.
TEA has been the traditional method of analgesia for postoperative pain management for pancreaticoduodenectomy as recommended by the ERAS [11]. It has more favorable outcomes than conventional parental opioids. It is associated with superior pain control and less postoperative ileus and pulmonary complications. Other ERAS-recommended analgesia modalities include TAWC and PCA. Inconsistences in their efficacies have been described, although both have shown comparable outcomes (to TEA) in analgesic and perioperative outcomes in recent studies [12,13]. Reasonably, both studies call for larger randomized trials to identify the best method of postoperative analgesia for pancreatic resection. To the best of our knowledge, this is the first study to investigate the use of IPA in pancreatic surgery. Existing studies comparing TEA and IPA are only available for non-pancreatic surgeries with mixed results. One study has reported superior analgesic properties and fewer complications with IPA in minimally invasive direct coronary artery bypass surgery [14], while others have suggested better pain control with TEA in post-thoracotomy and chest-wall trauma patients [7,15]. Similar outcomes have also been reported for these two analgesia modalities [16]. A variety of reasons can be responsible for these discrepancies, such as differences in the nature of operations, patient demographics, techniques of analgesia placement, and dosages of medication given, to name a few. Furthermore, it is difficult to predict the value of IPA in pancreatic surgery, based on the existing literature.
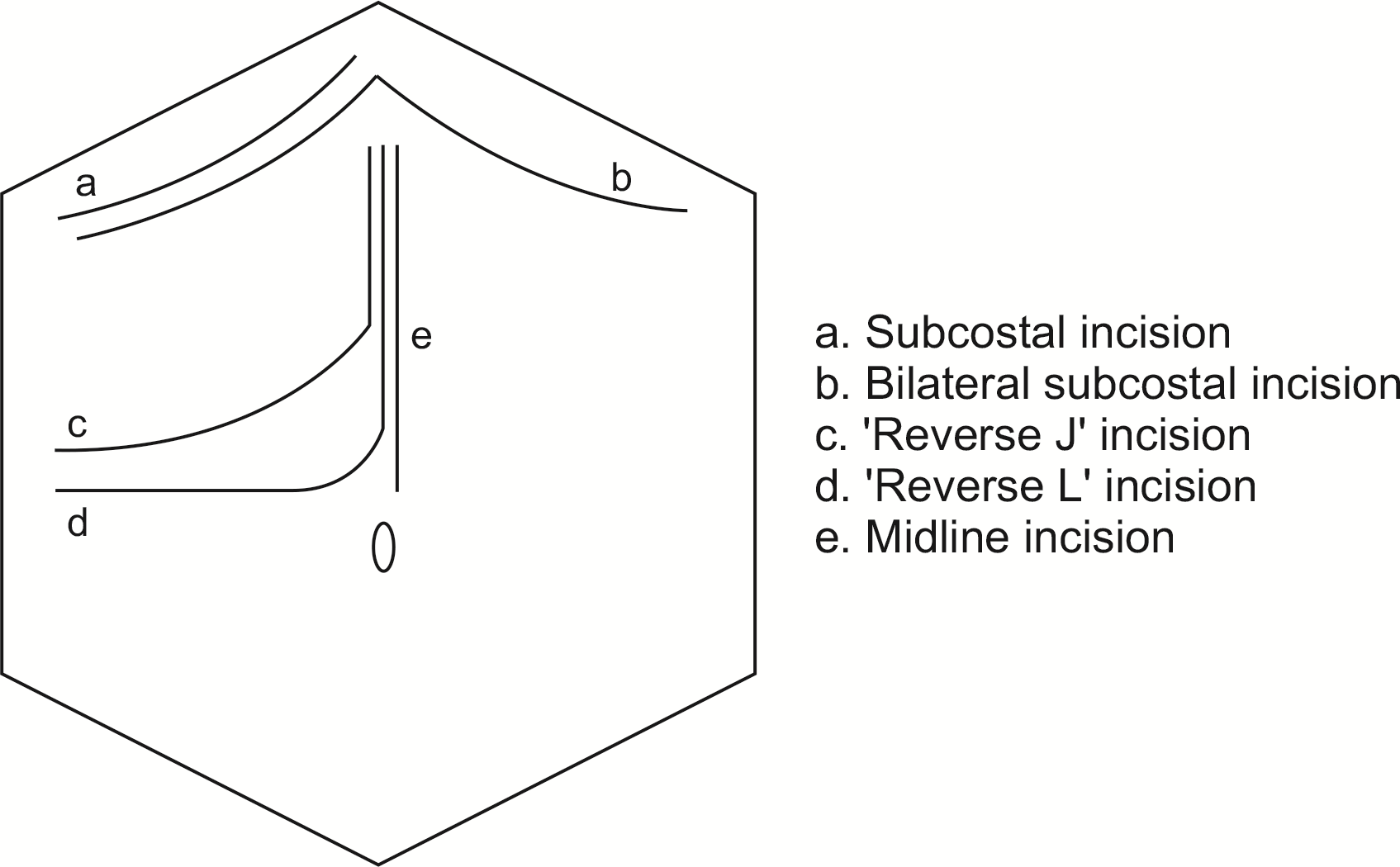
The choice of analgesia is also influenced by different ways of approaching the abdomen for access. KWPD can be performed via right subcostal, bilateral subcostal, midline, and ‘reverse J’, and ‘reverse L’ incisions (Fig. 1). A combination of vertical and horizontal incision created with ‘reverse L’ or ‘J’ incision techniques offer maximal exposure to the right upper quadrant of the abdomen. The ‘reverse L’ incision is preferred over the ‘reverse J’ incision as the horizontal limb of the latter is more proximal to the rib case, resulting in more pain with more associated complications [17,18]. For access to the pancreatic body or tail, a bilateral subcostal or midline incision is necessary. TEA would be a better option as it acts on spinal nerves bilaterally within the epidural space [19]. We acknowledge that IPA will not be sufficient as it provides unilateral control.
There are also disadvantages with TEA. Its association with hemodynamic instability has been well documented in the literature [10,11,19,20]. The main culprit is theorized to be the more significant sympathetic blockade in TEA, resulting in cardio-depressant effects and inhibition of vasoconstriction leading to functional hypovolaemia [10,20]. As inotropes are used to manage hemodynamic instability, our study used the extent of inotrope requirement as a loose indicator, showing comparable results to the literature. While the number of patients who had inotropes was similar between IPA and TEA groups, the latter group required significantly longer duration (median duration of 18 hours vs. 35 hours). Although the exact reason for this was unclear, it might be due to hemodynamic instability caused by TEA as the duration and type of surgery were similar between the two groups. Recent research has suggested that both inotropes and the duration of use have a deleterious compound relationship with pancreatic leaks [21]. Although the reason for this is unclear, we speculate that the timing of use is essential. The early postoperative period encompasses the inflammatory phase of wound healing consisting of influx of cytokines, leukocytes, and growth factors that can promote debris removal [22]. Hypotension with or without the use of inotropes can divert blood supply away from the superior mesenteric artery and microvascular flow, compromising anastomotic perfusion [23]. This suboptimal perfusion may result in persistent presence of bacteria and compromise the supply of oxygen and nutrients, subsequently leading to an anastomotic breakdown [24]. A shorter duration of inotrope use may be less consequential as early cessation will allow reperfusion and re-continuation of the healing process.
The logistic disadvantage with a prolonged ITUS associated with TEA (median of 3 vs. 2 days) is that it will increases costs and resource demands in the National Health Service (NHS), which costs approximately £1,500 per day [25]. This is even more relevant in the Covid-19 pandemic when there is a growing demand for ITU specialist care. It may be favorable for practitioners to consider IPA over TEA for suitable patients to lessen current strains.
The presented study has some limitations, including its small sample size and a single-centered retrospective study known to be associated with inherent biases. As resources and expertise vary across different trusts, our results might not be transferable across all hospitals and settings. We did not measure the amount or the type of medication used in PCA in each group. However, there was no reason to believe that PCA usage differed between the two groups. IPA also has drawbacks. One issue is that not all anesthetists are familiar with the technique. This, not every patient could have IPA despite its advantages. In our center, following review of IPA results, all consultant anesthetists would insert IPA for patients who undergo open pancreatic and liver surgeries through a ‘reverse L’, ‘one-sided subcostal’, or ‘reverse ‘J’ incision.
Despite these limitations, we presented our experience with a novel analgesic technique used in a cohort of patients operated by the same surgeon using a similar type of incision. We showed that IPA was not inferior to TEA in its efficacy of pain management for KWPD. Secondly, IPA (with or without PCA) had significantly fewer analgesia-related complications and inotropic requirements. In our unit, we now use IPA routinely. However, a randomized controlled study is needed to investigate the efficacy of IPA against other analgesic methods including TEA.
ACKNOWLEDGEMENTS
The authors thank Mr. Muhammed Abdalkoddus and Professor Shangming Zhou for assisting with the statistical analysis and the Plymouth University Hospital Hepto-Pancreatico-Biliary performance team for obtaining the patient list.
REFERENCES
1. White PF, Kehlet H, Neal JM, Schricker T, Carr DB, Carli F. 2007; The role of the anesthesiologist in fast-track surgery: from multimodal analgesia to perioperative medical care. Anesth Analg. 104:1380–1396. DOI: 10.1213/01.ane.0000263034.96885.e1. PMID: 17513630.
2. Manassero A, Bossolasco M, Carrega M, Coletta G. 2020; Postoperative thoracic epidural analgesia: adverse events from a single-center series of 3126 patients. Local Reg Anesth. 13:111–119. DOI: 10.2147/LRA.S272410. PMID: 32982397. PMCID: PMC7490049.
3. Phillips S, Dedic-Hagan J, Baxter DF, Van der Wall H, Falk GL. 2018; A novel technique of paravertebral thoracic and preperitoneal analgesia enhances early recovery after oesophagectomy. World J Surg. 42:1787–1791. DOI: 10.1007/s00268-017-4369-9. PMID: 29164294.
4. Cheng G, Ilfeld B. 2016; A review of postoperative analgesia for breast cancer surgery. Pain Manag. 6:603–618. DOI: 10.2217/pmt-2015-0008. PMID: 27481184.
5. Yu S, Valencia M, Roques V, Aljure O. 2019; Regional analgesia for minimally invasive cardiac surgery. J Card Surg. 34:1289–1296. DOI: 10.1111/jocs.14177. PMID: 31441548.
6. Murphy D. 1993; Interpleural analgesia. Br J Anaesth. 71:426–434. DOI: 10.1093/bja/71.3.426. PMID: 8398528.
7. Yildirim V, Akay H, Bingol H, Bolcal C, Oz K, Kaya E, et al. 2007; Interpleural versus epidural analgesia with ropivacaine for postthoracotomy pain and respiratory function. J Clin Anesth. 19:506–511. DOI: 10.1016/j.jclinane.2007.04.005. PMID: 18063204.
8. Dhanjal S, Shannon C. 2021. Interpleural analgesia [Internet]. StatPearls Publishing;Treasure Island: Available from: https://www.ncbi.nlm.nih.gov/books/NBK526020/. cited 2021 Jan 14.
9. Regional Anaesthesia-UK. 2011. Stop before you block [Internet]. Regional Anaesthesia-UK;London: Available from: https://www.ra-uk.org/index.php/stop-before-you-block. cited 2021 Sep 6.
10. Manion S, Brennan T. 2011; Thoracic epidural analgesia and acute pain management. Anesthesiology. 115:181–188. DOI: 10.1097/ALN.0b013e318220847c. PMID: 21606825.
11. Lassen K, Coolsen M, Slim K, Carli F, de Aguilar-Nascimento JE, Schäfer M, et al. 2013; Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS®) society recommendations. World J Surg. 37:240–258. DOI: 10.1007/s00268-012-1771-1. PMID: 22956014.
12. Akter N, Ratnayake B, Joh DB, Chan SJ, Bonner E, Pandanaboyana S. 2021; Postoperative pain relief after pancreatic resection: systematic review and meta-analysis of analgesic modalities. World J Surg. 45:3165–3173. DOI: 10.1007/s00268-021-06217-x. PMID: 34185150. PMCID: PMC8408074.
13. Perrin J, Ratnayake B, Wells C, Windsor JA, Loveday BPT, MacLennan N, et al. 2021; Epidural versus transabdominal wall catheters: a comparative study of outcomes after pancreatic resection. J Surg Res. 259:473–479. DOI: 10.1016/j.jss.2020.09.005. PMID: 33070995.
14. Mehta Y, Swaminathan M, Mishra Y, Trehan N. 1998; A comparative evaluation of intrapleural and thoracic epidural analgesia for postoperative pain relief after minimally invasive direct coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 12:162–165. DOI: 10.1016/S1053-0770(98)90324-X. PMID: 9583546.
15. Luchette F, Radafshar S, Kaiser R, Flynn W, Hassett J. 1994; Prospective evaluation of epidural versus intrapleural catheters for analgesia in chest wall trauma. J Trauma. 36:865–869. discussion 869–870. DOI: 10.1097/00005373-199406000-00018. PMID: 8015010.
16. Brockmeier V, Moen H, Karlsson B, Fjeld N, Reiestad F, Steen P. 1994; Interpleural or thoracic epidural analgesia for pain after thoracotomy. A double blind study. Acta Anaesthesiol Scand. 38:317–321. DOI: 10.1111/j.1399-6576.1994.tb03900.x. PMID: 8067216.
17. Chang S, Palavecino M, Wray C, Kishi Y, Pisters PW, Vauthey JN. 2010; Modified Makuuchi incision for foregut procedures. Arch Surg. 145:281–284. DOI: 10.1001/archsurg.2010.7. PMID: 20231630.
18. Pandit N, Awale L, Adhikary S, Banerjee JK, Ghosh S, Kulkarni S, et al. 2019; Modified Makuuchi incision for major upper abdominal surgeries. Pol Przegl Chir. 91:15–19. DOI: 10.5604/01.3001.0013.5382. PMID: 31849352.
19. Toledano RD, Van de Velde M. 2017. Epidural anesthesia and analgesia [Internet]. NYSORA;New York: Available from: https://www.nysora.com/regional-anesthesia-for-specific-surgical-procedures/abdomen/epidural-anesthesia-analgesia/. cited 2021 Sep 7.
20. Clemente A, Carli F. 2008; The physiological effects of thoracic epidural anesthesia and analgesia on the cardiovascular, respiratory and gastrointestinal systems. Minerva Anestesiol. 74:549–563. PMID: 18854796.
21. Casey P, Chaudhury MP, Khan A, Amin J, Afzal A, Corallo C, et al. 2019; The impact of perioperative inotropes on the incidence of pancreatic leak following pancreaticoduodenectomy. Ann Hepatobiliary Pancreat Surg. 23:392–396. DOI: 10.14701/ahbps.2019.23.4.392. PMID: 31825007. PMCID: PMC6893053.
22. Williams N, O'Connell P, McCaskie A. Bailey & Love's short practice of surgery. 27th ed. Boca Raton: CRC Press;2018.
23. Spronk P, Zandstra D, Ince C. 2004; Norepinephrine compromises intestinal microvascular perfusion? Intensive Care Med. 30:173–174. author reply 175DOI: 10.1007/s00134-003-2067-6. PMID: 14647887.
24. Guo S, Dipietro LA. 2010; Factors affecting wound healing. J Dent Res. 89:219–229. DOI: 10.1177/0022034509359125. PMID: 20139336. PMCID: PMC2903966.
25. British Broadcasting Corporation. 2010. Intensive care 'disaster' warning [Internet]. BBC;London: Available from: https://www.bbc.co.uk/news/health-11503873. cited 2021 Mar 19.
Table 1
Patient demographics, co-morbidities, peri-operative details, post-operative complications and duration of stay
Table 2
Frequency of daily reported pain severity between POD 0–5
Table 3
Sum of the frequency of adequate versus inadequate pain control between POD 0–3
| Severity of pain | TEA (n = 85) | IPA (n = 69) | p-value |
|---|---|---|---|
| Adequatea) | 74 (87.1) | 52 (75.4) | 0.097 |
| None | 32 (37.6) | 27 (39.1) | 0.983 |
| Mild | 42 (49.4) | 25 (36.2) | 0.140 |
| Inadequateb) | 11 (12.9) | 17 (24.6) | 0.097 |
| Moderate | 10 (11.8) | 15 (21.7) | 0.147 |
| Severe | 1 (1.2) | 2 (2.9) | 0.587 |
Table 4
Analgesia-related complications, respiratory-related complications and inotrope requirements
| Variable | TEA (n = 22) | IPA (n = 18) | p-value |
|---|---|---|---|
| Analgesia-related complication | 8 | 1 | 0.027 |
| Not working | 4 | 0 | 0.114 |
| Leakage | 2 | 1 | > 0.999 |
| Refractory haemodynamic instabilitya) | 1 | 0 | > 0.999 |
| Lower limb paraesthesia | 1 | 0 | > 0.999 |
| Respiratory-related complication | |||
| Hospital acquired pneumonia | 7 | 6 | > 0.999 |
| Use of inotrope | |||
| Inotrope requirement | 11 | 8 | 0.975 |
| Median duration of inotrope use (h) | 35 | 18 | 0.047 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download