Abstract
Backgrounds/Aims
Gallbladder cancer (GBC) is a rare neoplasm. The epidemiology of GBC has not been updated in Australia for over five decades.
Methods
Data of all Australian patients diagnosed with GBC at any age from 1982 to 2018 were identified from the Australian Cancer Database. Age-standardized rates were calculated and joinpoint analysis was performed to ascertain the trends of incidence and mortality of GBC.
Results
Between 1982 and 2018, there were 22,745 cases of GBC and 11,054 GBC-related deaths in Australia. There were three distinct periods showing changed incidence. Period 1 (1982–1995) was stable. Period 2 (1996–2006) showed reduced incidence in females (3.6 to 2.8/100,000; p < 0.01) and all Australians (3.7 to 2.8/100,000, p < 0.01). Period 3 (2006–2017) demonstrated significantly increased incidence in all groups (males: 2.7 to 4.0/100,000, p < 0.01; females: 2.8 to 3.5/100,000, p < 0.01; all Australians: 2.8 to 3.7/100,000, p < 0.01). Incidence between females and males had declined from 1.10 : 1 in 1982 to 0.87 : 1 in 2017. There was a 71% reduction in mortality (3.1 to 0.9/100,000; p < 0.01). Median age at diagnosis increased from 69.7 to 74.3 years for females and from 67.2 to 73.3 years for males. Increasing incidence in the 6th to 8th decade of life in males, compared to previous years, was noted.
Gallbladder cancer (GBC) is a rare neoplasm with a poor prognosis. It accounts for about 1.3% of total cancer incidence worldwide and 1.7% of all cancer deaths [1]. The first study documenting GBC in Australia was published in 1965 [2]. It investigated 56 episodes over a five-year period [2]. Whilst incidence and mortality of GBC have been investigated worldwide including in the United States (USA) [3], South Korea [4], New Zealand [5], Canada [6], and the United Kingdom (UK) [7], there has been no published literature on the incidence or mortality of GBC in Australia since 1965.
Decades later, GBC remains poorly understood, with a complex interplay of genetic, lifestyle, infectious, and metabolic factors contributing to carcinogenesis. Incidence of GBC has also been noted to exhibit wide geographic variations between developed and developing nations. Unlike most other cancers, GBC demonstrates a strong deviation towards female patients, with female-to-male ratio of 1.1 to 2.6 [8].
Therefore, updating our understanding of GBC is essential. This study aims to contribute to the knowledge base by describing contemporary trends in the incidence and mortality of GBC in Australia and to assess these trends with regards to age, sex, and geography. By identifying temporal trends of GBC incidence, we aim to guide future research in identifying risk factors or preventative factors for periods showing shifting incidence of GBC.
The Australian Cancer Database (ACD) is a nationwide database documenting all new cases of cancer diagnoses and deaths in Australia since January 1st, 1982 [9]. The ACD is maintained by the Australian Institute of Health and Welfare (AIHW) [10] who are responsible for auditing and standardizing the datum. The ACD records data for all Australian patients diagnosed with GBC from 1982 to 2018 by age (2-year intervals), sex, and state. Factors related to histopathology, ethnicity, and surgical intervention are not reported. Data from this Australian population cohort were collected and analyzed.
Age-specific rates for incidence and mortality rate per 100,000 population were calculated and then standardized for age using the direct age-standardization method. The Australian population on June 30, 2001 was used as the standard population to determine the age-standardized rate (ASR). Annual percent changes (APCs) for incidence and mortality were calculated using joinpoint regression analysis [11]. This involves a series of joined straight lines being fit on a logarithmic scale to trends in the annual age-adjusted cancer incidence and mortality rates. The aim of joinpoint analysis is to determine whether a multi-segmented line is a significantly better fit than a straight or less-segmented line for a set of data. A two-slope t-test was then used to determine if APCs of these lines represented a statistically significant change (either increase or decrease) in rate. Significance was defined at p-value < 0.05. Any changes in APCs that were not statistically significant were considered stable. Our analysis determined three distinct time periods in changing GBC incidence in Australia. The 95% confidence intervals (95% CI) were obtained with a standard error from the fit of the regression and the t-distribution function. Median age at diagnosis of GBC was analyzed using univariable linear regression. All statistical analyses were completed using GraphPad Prism v8.4 (GraphPad Software Inc., San Diego, CA, USA), Stata v17.0 (StataCorp, College Station, TX, USA), and Joinpoint Trend Analysis Software v4.9 (Statistical Research and Applications Branch, National Cancer Institute, Bethesda, MD, USA). State-specific incidence for the Northern Territory (NT) was available only after 2009. The Australian Capital Territory (ACT) was excluded from our analysis due to unavailability of data.
Between 1982 and 2018, there were 22,745 cases of GBC and 11,054 GBC-related deaths. Over the last four decades (1980s, 1990s, 2000s, 2010s), incidence for males had shifted from 3.2 to 3.4 to 2.9 and 3.5 per 100,000, respectively. For females, this change has been from 3.6 to 3.8 to 3.0 and 3.3 per 100,000, respectively. The female to male ASR ratio was 1.10 : 1 in 1982 and 0.87 : 1 in 2017. Amongst individual states, in the 1980s and 1990s (Fig. 1A, 1B), Victoria (VIC) had the highest observed incidence of GBC (3.7 to 3.8 per 100,000), followed by Queensland (QLD) (3.4 to 3.6 per 100,000) and New South Wales (NSW) (3.2 to 3.4 per 100,000). In the subsequent decade (Fig. 1C), the incidence of GBC dropped in all states. In the next decade (2010’s), incidence rose in NSW (2.7 to 3.3 per 100,000), QLD (3.0 to 3.4 per 100,000), and Western Australia (3.3 to 3.6 per 100,000). It remained stable in VIC (3.1 per 100,000) and South Australia (SA) (3.4 to 3.5 per 100,000). Complete data from the NT were only available for the 2010s where the ASR was higher than all other states at 6.8 per 100,000, nearly double that of the next state, SA, at 3.5 per 100,000 (Fig. 1D). ACT data were not available.
The median age at diagnosis of GBC had increased in Australia between 1982 and 2017: from 69.7 years to 74.3 years in females (r2 = 0.76, β = 0.13; p < 0.01) and from 67.2 years to 73.3 years in males (r2 = 0.76, β = 0.15; p < 0.01) (Fig. 2A). The age-demographic for peak incidence had also shifted from the 9th decade of life for males in 1982 to the 8th decade in 2000 and 2017 (Fig. 2B, 2C). There was also a progressive rise in GBC incidence from ages 40–80 years in 2017 than in 2000 and 1982 (Fig. 2C). Peak incidence has remained stable in the 8th decade of life (Fig. 2B, 2D) for females, although it showed a sharp drop in the 9th decade, unlike findings for males.
Fig. 3 demonstrates the incidence rate for GBC in Australia from 1982 to 2017. Table 1 reports data from joinpoint analysis. Our joinpoint analysis identified three distinct time periods showing changed GBC incidence in Australia (each period was ± 2 years depending on sub-population analysed): period 1 (1982–1996/1998), period 2 (1996/1998–2003/2005), and period 3 (2003/2005–2017). Males (ASR 3.3 to 3.5, APC 0.70%; p = 0.14), females (ASR 3.6 to 3.6, APC -0.40%; p = 0.11), and all Australians (ASR 3.5 to 3.7, APC 0.10%; p = 0.84) in period 1 demonstrated no statistically significant change in GBC incidence. Period 2 showed a statistically significant decline in incidence amongst females (ASR 3.6 to 2.8, APC -3.20%; p < 0.01) and all Australians (ASR 3.7 to 2.8, APC -2.80%; p < 0.01). Due to statistical significance being defined at p < 0.05, the trend for period 2 was considered stable amongst males (ASR 3.5 to 2.7, APC -4.80%; p = 0.07) despite the largest decline in GBC incidence over this period. In the final period, incidence amongst all groups showed a significant increase. The incidence increased from 2.7 to 4.0 in males (APC 2.60%, p < 0.01), from 2.8 to 3.5 in females (APC 1.70%, p < 0.01), and from 2.8 to 3.7 in all Australians (APC 2.20%, p < 0.01).
Mortality showed a near linear reduction (Fig. 4) in Australia with a reduction from 3.1 to 0.9 per 100,000 (1971 to 2019), a 71% decrease (r2 = 0.95, β = -0.05; p < 0.01). In the same period, male-specific mortality decreased from 2.7 to 0.8 per 100,000 (r2 = 0.85, β = -0.04; p < 0.01) and female-specific mortality decreased from 3.4 to 1.0 per 100,000 (r2 = 0.93, β = -0.05; p < 0.01). Joinpoint regression demonstrated an average APC in mortality of -2.6% (95% CI: -4.2% to -0.9%; p < 0.05).
Our study showed that the incidence of GBC had notable shifts over the time period analyzed; initially steady between 1982 to 1997, a decline from 1998 to 2005, followed by a significant increase from 2006 to 2017. The median age at time of diagnosis for both sexes has also increased in a linear fashion since 1982, with males typically diagnosed earlier than females. Across 35 years, the peak incidence of disease had remained stable for males and females. State-specific incidence rate exhibited some variations over the last four decades, with declining incidence in VIC and rising incidence in the NT.
The incidence of GBC in Australia reached its nadir in 2006. The decline between 1998 and 2006 might be partially explained by an increase in the uptake of cholecystectomy in Australia, particularly after the introduction of laparoscopic cholecystectomy in circa 1990 [12]. Per AIHW data, total cholecystectomies (public and private) in Australia demonstrated a 6.5% increase, from 205.5 to 218.7 per 100,000 population between 2000 and 2019 [13]. Furthermore, peak incidence of cholecystectomy shifted from age 45–54 years in 2000 to 35–44 years in 2016. Of note, the median age of diagnosis for GBC shown in our study was 67–73 years old. It is known that the duration between metaplasia to cancer-in-situ for GBC is up to 20 years [14], indicating that metaplasia might begin to develop between 47–53 years old. We hypothesize that the shift to a younger demographic undergoing cholecystectomy resulting in removal of the gallbladder earlier in life might have contributed to shifting GBC incidence and mortality. The link between cholecystectomy and an observed reduction in GBC mortality has been suggested as early as 1981 [15]. A decrease in cholecystectomy rate seen in Chile in the 1980s and 1990s has also been suggested as a cause for a rise in GBC incidence and tripling in mortality over that time period [16,17].
However, the cause for increase in incidence in Australia after 2006 is not entirely clear. GBC remains a relatively obscure cancer with wide geographic and sex variations. The highest incidences worldwide have been reported amongst female in Delhi, India (21.5/100,000), South Karachi, Pakistan (13.8/100,000), and Quito, Ecuador (12.9/100,000) [18], three to five-fold higher than that those seen in Australia. In these areas, suggested risk factors include infection (chronic Salmonella typhi and Helicobacter pylori), exposure to environmental pollutants and heavy metals [17], limited access to care, and malnutrition [19]. Modifiable risk factors common to all developed nations include diabetes, smoking, obesity, and hypercholesterolemia. However, incidence rates amongst high income/developed nations also show variability. South Korean data from 1999 to 2013 demonstrated similar incidence compared to Australia (3.0 vs. 3.1/100,000) [4]. Considerably lower cases (1.1 vs. 3.1/100,000) in the USA between 2007 and 2011 have been reported [3]. Canadian data demonstrated higher rates than Australia (11.4 vs. 3.1/100,000) in 2012 [6], whereas UK data [7] showed lower rates (1.8 vs. 3.1/100,000) in 2016 to 2018. Although there is potential for significant variation in GBC incidence amongst developed nations, we are unable to rule out large differences due to deviations in methods used to calculate ASR. GBC’s unique predilection for females also seems to have shifted in Australia after 2014, with incidence in females now being 12.5% lower than that in males. This was contrary to results from the 2018 Global Cancer Observatory (GLOBOCAN) database [20] which demonstrated an ongoing high incidence amongst females. It may be interesting to assess if cholecystectomy rates amongst females are higher than in males to explain the changing incidence. No obvious risk factors for GBC exist in developed nations, although gallstones have been previously implicated [21]. This might have contributed to the increase of Australian GBC incidence rate given a progressively overweight and older Australian population [22], both known to be linked to gallstone formation. The epidemiology of gallstone disease in Australia has not been recently studied. Thus, this is speculative.
Our results revealed that the NT had the highest rate of GBC. In 2016, the Aboriginal and Torres Strait Islander (ATSI) population made up approximately 30% of the state’s population compared to 3.3% across Australia [23]. Although the lack of granularity in our dataset limits us from being able to link diagnoses of GBC to specific populations, previous evidence has demonstrated that the incidence of GBC amongst Indigenous Australians is as high as 30/100,000 [24], eight-fold higher than that in the general population. This could be related to the higher prevalence of chronic viral hepatitis [25] amongst the ATSI population, which is associated with an increased risk of extrahepatic cancer [26,27]. Other factors include higher overall cancer incidence and mortality rate amongst the ATSI population, fewer cancer-related hospitalizations and decreased healthcare access, and higher prevalence of cancer-related modifiable risk factors [28].
Mortality also showed a significant decline of 71% in Australia over the last 35 years. This is substantially better than other worldwide estimates. The 2018 GLOBOCAN data published an estimated worldwide mortality rate of 1.6/100,000 for males and 1.8/100,000 for females (compared to 0.9 [males] and 1.0 [females] in Australia) [29]. This is likely attributable to the advanced healthcare system in Australia which facilitates early diagnosis, advanced surgical techniques (liver resection and lymph node dissection), and progressive adjuvant therapy. Although survival data were not available for analysis in our study, single institution data from an Australian tertiary referral hospital demonstrated a median survival of 35 months [29] for GBC patients treated at their center.
This study provides a comprehensive overview of GBC trends in Australia from 1982 to 2018 using the best available insights into Australian cancer statistics. However, it has several limitations. Unlike other national studies [3,4,6], we were unable to identify the histological subtype of GBC diagnoses as they were not reported in AIHW data. Furthermore, this was a national database review that relied on the granularity and accuracy of ACD and AIHW results. As the aforementioned databases are not patient registries, we were unable to link diagnoses to individual patients. Thus, we were not able to assess additional demographic information, risk factors, staging, treatment, or survival outcomes. This limits our ability to gain further insights into the state of GBC in Australia. Nevertheless, this method of analysis has been validated in studies on hepatocellular carcinoma [30,31], colorectal cancer [32], and appendiceal cancer [33] in Australia.
In conclusion, over the last 35 years, the incidence, mortality, sex, and age demographics of GBC in Australia have exhibited shifts. A steady rise in GBC incidence since 2006 is of concern and warrants further investigation and should be a focus of future research. We concede several limitations of our study. Analyses of trends require careful interpretation as changes in prevalence of risk factors, demography/immigration, diagnostic techniques, and screening tools are reflected in the changes of incidence. With an aging population, the overall burden of GBC is predicted to increase in the future. Nevertheless, the mortality rate from GBC in Australia continued a downward trend. This is a promising finding. Further longitudinal studies are needed to gain insight into the epidemiological trends, etiology, and prognosis of GBC.
REFERENCES
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. 2015; Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. DOI: 10.1002/ijc.29210. PMID: 25220842.
2. Bennett RC, Jepson RP. 1965; Carcinoma of the gall-bladder. Aust N Z J Surg. 34:278–283. DOI: 10.1111/j.1445-2197.1965.tb04375.x. PMID: 14296752.
3. Henley SJ, Weir HK, Jim MA, Watson M, Richardson LC. 2015; Gallbladder cancer incidence and mortality, United States 1999-2011. Cancer Epidemiol Biomarkers Prev. 24:1319–1326. DOI: 10.1158/1055-9965.EPI-15-0199. PMID: 26070529.
4. Wi Y, Woo H, Won YJ, Jang JY, Shin A. 2018; Trends in gallbladder cancer incidence and survival in Korea. Cancer Res Treat. 50:1444–1451. DOI: 10.4143/crt.2017.279. PMID: 29370591. PMCID: PMC6192934.
5. Koea J, Phillips A, Lawes C, Rodgers M, Windsor J, McCall J. 2002; Gall bladder cancer, extrahepatic bile duct cancer and ampullary carcinoma in New Zealand: demographics, pathology and survival. ANZ J Surg. 72:857–861. DOI: 10.1046/j.1445-2197.2002.02589.x. PMID: 12485219.
6. Flemming JA, Zhang-Salomons J, Nanji S, Booth CM. Increased incidence but improved median overall survival for biliary tract cancers diagnosed in Ontario from 1994 through 2012: a population-based study. Cancer. 2016; 122:2534–2543. DOI: 10.1002/cncr.30074. PMID: 27183133.
7. Cancer Research UK. 2015. Gallbladder cancer incidence statistics [Internet]. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/gallbladder-cancer/incidence. cited 2021 Oct 10. London: Cancer Research UK.
8. Torre LA, Siegel RL, Islami F, Bray F, Jemal A. 2018; Worldwide burden of and trends in mortality from gallbladder and other biliary tract cancers. Clin Gastroenterol Hepatol. 16:427–437. DOI: 10.1016/j.cgh.2017.08.017. PMID: 28826679.
9. Australian Institute of Health and Welfare. 2021. Australian Cancer Database (ACD) [Internet]. Available from: https://www.aihw.gov.au/about-our-data/our-data-collections/australian-cancer-database. cited 2021 Sep 27. Darlinghurst: Australian Institute of Health and Welfare.
10. Australian Institute of Health and Welfare. 2021. Cancer data in Australia [Internet]. Available from: https://www.aihw.gov.au/reports/cancer/cancer-data-in-australia/contents/summary. cited 2021 Sep 25. Darlinghurst: Australian Institute of Health and Welfare.
11. Kim HJ, Fay MP, Feuer EJ, Midthune DN. 2000; Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 19:335–351. Erratum in: Stat Med 2001;20:655. DOI: 10.1002/sim.811. PMID: 10649300.
12. Hardy KJ. 2011; Laparoscopic cholecystectomy: Australian beginnings. ANZ J Surg. 81:866–870. DOI: 10.1111/j.1445-2197.2011.05898.x. PMID: 22507410.
13. Australian Institute of Health and Welfare. 2021. Procedures data cubes [Internet]. Available from: https://www.aihw.gov.au/reports/hospitals/procedures-data-cubes/contents/data-cubes. cited 2021 Oct 8. Darlinghurst: Australian Institute of Health and Welfare.
14. Hundal R, Shaffer EA. 2014; Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 6:99–109. DOI: 10.2147/CLEP.S37357. PMID: 24634588. PMCID: PMC3952897.
15. Diehl AK, Beral V. 1981; Cholecystectomy and changing mortality from gallbladder cancer. Lancet. 2:187–189. DOI: 10.1016/S0140-6736(81)90366-4. PMID: 6114251.
16. Serra I, Yamamoto M, Calvo A, Cavada G, Báez S, Endoh K, Watanabe H, Tajima K. 2002; Association of chili pepper consumption, low socioeconomic status and longstanding gallstones with gallbladder cancer in a Chilean population. Int J Cancer. 102:407–411. Erratum in: Int J Cancer 2003;104:798. DOI: 10.1002/ijc.10716. PMID: 12402311.
17. Lee MH, Gao YT, Huang YH, McGee EE, Lam T, Wang B, et al. 2020; A metallomic approach to assess associations of serum metal levels with gallstones and gallbladder cancer. Hepatology. 71:917–928. DOI: 10.1002/hep.30861. PMID: 31318976. PMCID: PMC6980252.
18. Randi G, Franceschi S, La Vecchia C. 2006; Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer. 118:1591–1602. DOI: 10.1002/ijc.21683. PMID: 16397865.
19. Dutta U, Bush N, Kalsi D, Popli P, Kapoor VK. 2019; Epidemiology of gallbladder cancer in India. Chin Clin Oncol. 8:33. DOI: 10.21037/cco.2019.08.03. PMID: 31484488.
20. Rawla P, Sunkara T, Thandra KC, Barsouk A. 2019; Epidemiology of gallbladder cancer. Clin Exp Hepatol. 5:93–102. DOI: 10.5114/ceh.2019.85166. PMID: 31501784. PMCID: PMC6728871.
21. Lowenfels AB, Walker AM, Althaus DP, Townsend G, Domellöf L. 1989; Gallstone growth, size, and risk of gallbladder cancer: an interracial study. Int J Epidemiol. 18:50–54. DOI: 10.1093/ije/18.1.50. PMID: 2722383.
22. Australian Institute of Health and Welfare. 2020. Overweight and obesity [Internet]. Available from: https://www.aihw.gov.au/reports/australias-health/overweight-and-obesity. cited 2021 Oct 2. Darlinghurst: Australian Institute of Health and Welfare.
23. Australian Bureau of Statistics. 2018. Estimates of Aboriginal and Torres Strait Islander Australians [Internet]. Available from: https://www.abs.gov.au/statistics/people/aboriginal-and-torres-strait-islander-peoples/estimates-aboriginal-and-torres-strait-islander-australians/latest-release. cited 2021 Oct 3. Belconnen: Australian Bureau of Statistics.
24. Zhang X, Condon J, Dempsey K, Garling L. Cancer in the Northern Territory 1991-2010: incidence, mortality and survival. Darwin: Department of Health;2014. DOI: 10.1093/ije/18.1.50.
25. Wigg AJ, Narayana SK, Hartel G, Medlin L, Pratt G, Powell EE, et al. 2021; Hepatocellular carcinoma amongst Aboriginal and Torres Strait Islander peoples of Australia. EClinicalMedicine. 36:100919. DOI: 10.1016/j.eclinm.2021.100919. PMID: 34142069. PMCID: PMC8187829.
26. Kamiza AB, Su FH, Wang WC, Sung FC, Chang SN, Yeh CC. 2016; Chronic hepatitis infection is associated with extrahepatic cancer development: a nationwide population-based study in Taiwan. BMC Cancer. 16:861. DOI: 10.1186/s12885-016-2918-5. PMID: 27821099. PMCID: PMC5100218.
27. Hsing AW, Zhang M, Rashid A, McGlynn KA, Wang BS, Niwa S, et al. 2008; Hepatitis B and C virus infection and the risk of biliary tract cancer: a population-based study in China. Int J Cancer. 122:1849–1853. DOI: 10.1002/ijc.23251. PMID: 18076042.
28. DiGiacomo M, Davidson PM, McGrath SJ, Dharmendra T, Thompson SC. 2012. Cancer in aboriginal and torres strait islander peoples: a rapid review of the literature. Sydney: UTS ePress;DOI: 10.1002/ijc.23251.
29. Wietsma MFT, Molloy C, Bhimani N, de Savornin Lohman EAJ, Gill AJ, Andrici J, et al. 2021; Gallbladder carcinoma outcomes in an Australian tertiary referral hospital. ANZ J Surg. 91:603–608. DOI: 10.1111/ans.16663. PMID: 33604992.
30. Cocker F, Chien Yee K, Palmer AJ, de Graaff B. 2019; Increasing incidence and mortality related to liver cancer in Australia: time to turn the tide. Aust N Z J Public Health. 43:267–273. DOI: 10.1111/1753-6405.12889. PMID: 30958629.
31. Wallace MC, Preen DB, Short MW, Adams LA, Jeffrey GP. Hepatocellular carcinoma in Australia 1982-2014: increasing incidence and improving survival. Liver Int. 2019; 39:522–530. DOI: 10.1111/liv.13966. PMID: 30230194.
32. Young JP, Win AK, Rosty C, Flight I, Roder D, Young GP, et al. 2015; Rising incidence of early-onset colorectal cancer in Australia over two decades: report and review. J Gastroenterol Hepatol. 30:6–13. DOI: 10.1111/jgh.12792. PMID: 25251195.
33. Mikaeel RR, Young JP, Hardingham JE, Tapia Rico G, Hewett PJ, Symonds EL, et al. 2021; Appendiceal neoplasm incidence and mortality rates are on the rise in Australia. Expert Rev Gastroenterol Hepatol. 15:203–210. DOI: 10.1080/17474124.2021.1832467. PMID: 33022181.
Fig. 1
Incidence of gallbladder cancer (GBC) in Australia per 100,000 population by decade. (A) 1980s (1982–1989), (B) 1990s (1990–1999), (C) 2000s (2000–2009), and (D) 2010s (2010–2017). Data for the Northern Territory were not recorded in the 1980s or 1990s.
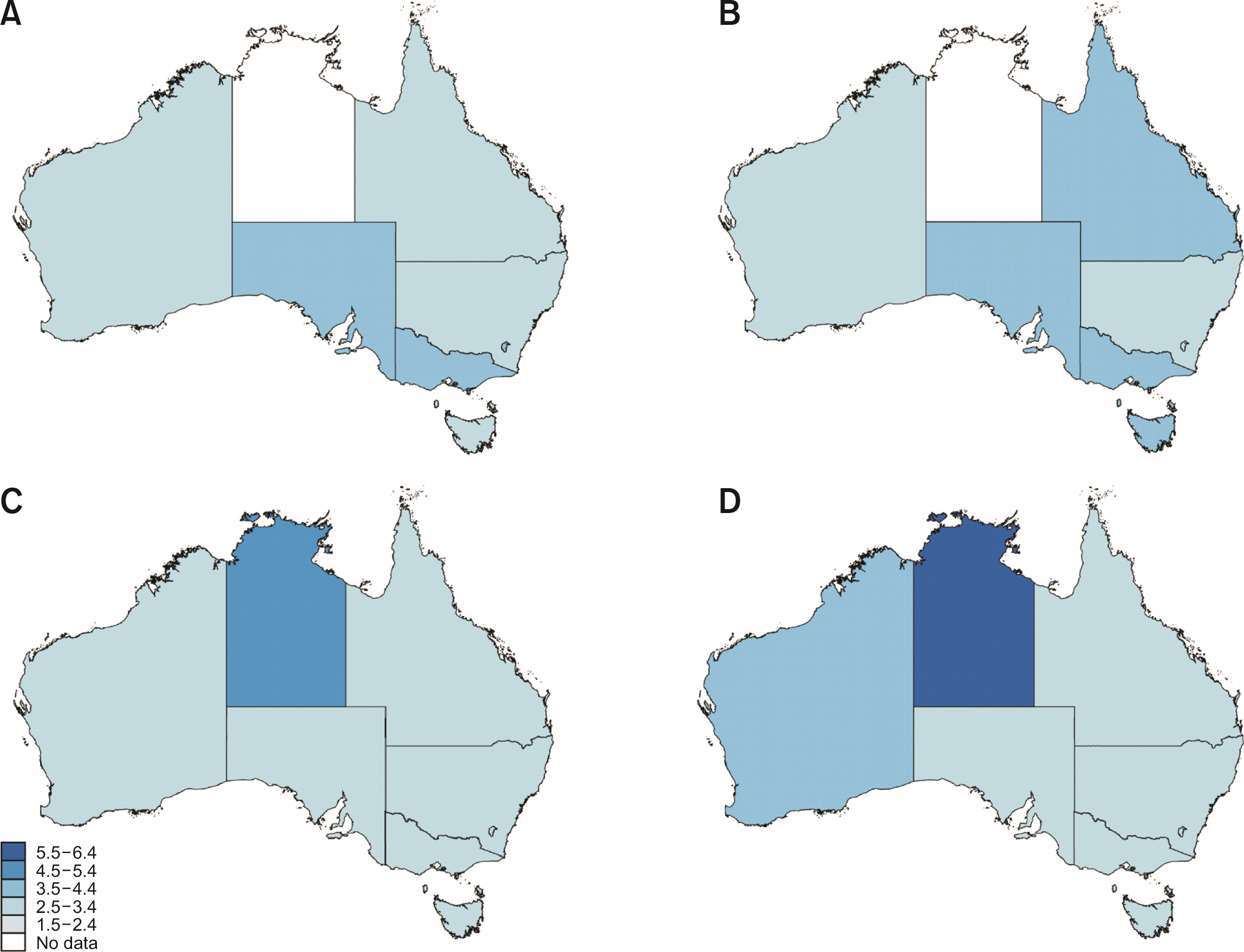
Fig. 2
Age demographics for gallbladder cancer in Australia. (A) Median age at time of diagnosis by sex with linear regression; (B) Age distribution in 1982 and 2017 by sex and age distribution (per 100,000 population) at three points in our dataset (1982, 2000, and 2017) for (C) males and (D) females.
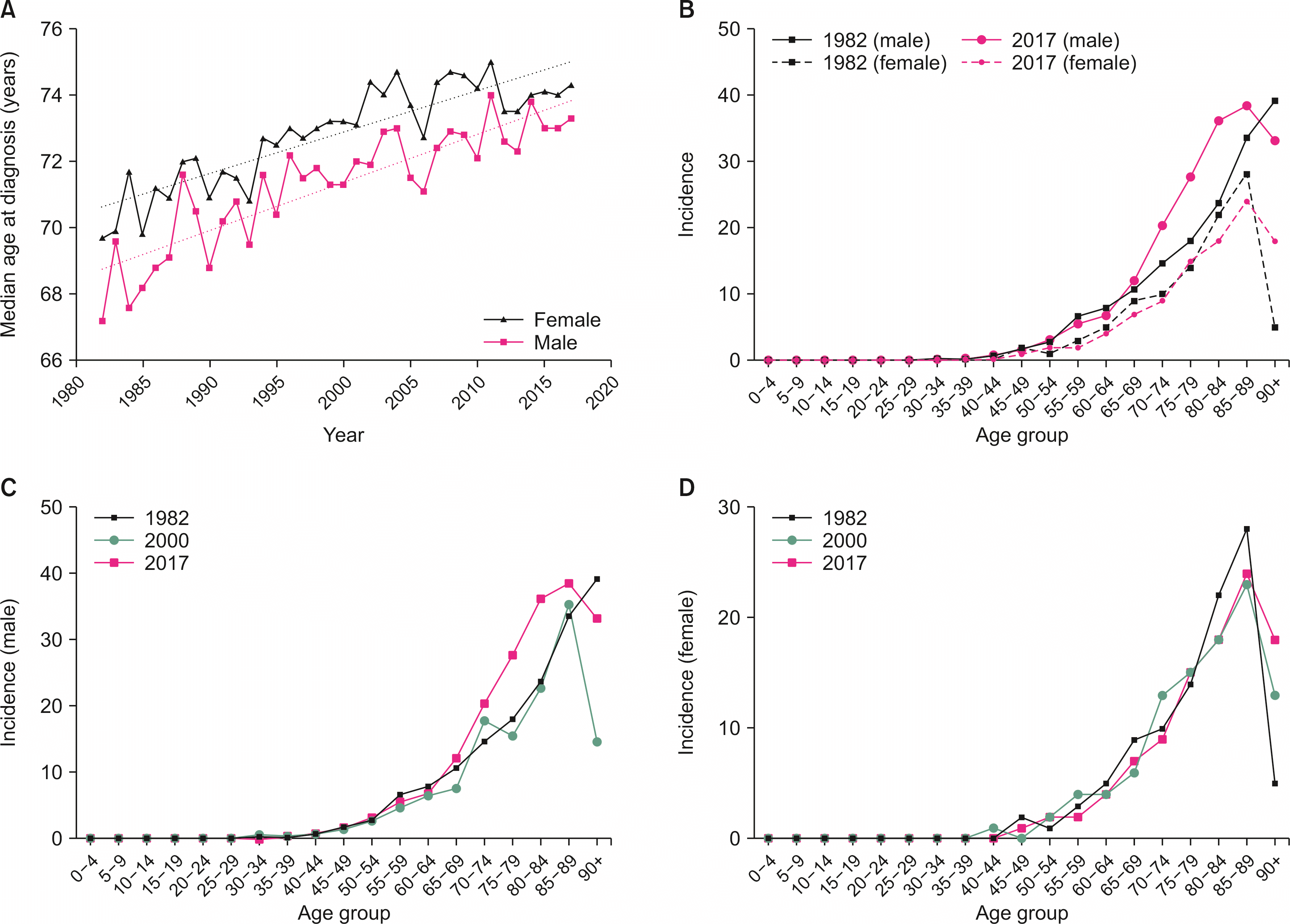
Fig. 3
Joinpoint regression analysis demonstrating three distinct periods showing changed Australian gallbladder cancer incidence (per 100,000 age-standardized population) separated by all Australians (A), females (B), and males (C).
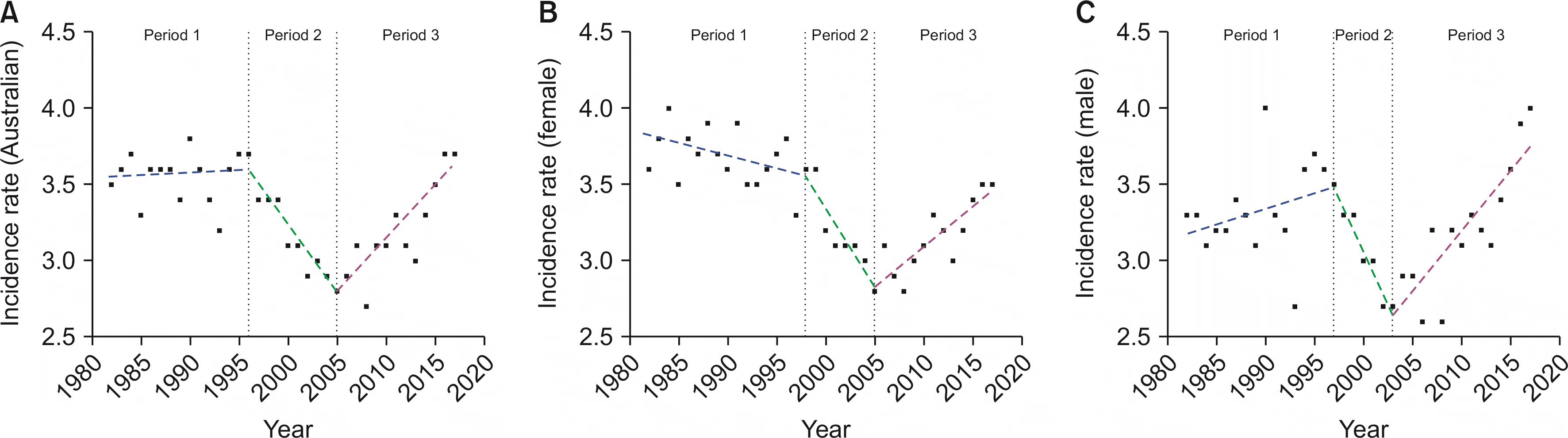
Fig. 4
Australian gallbladder cancer mortality rate (per 100,000 age-standardized population) according to sex.
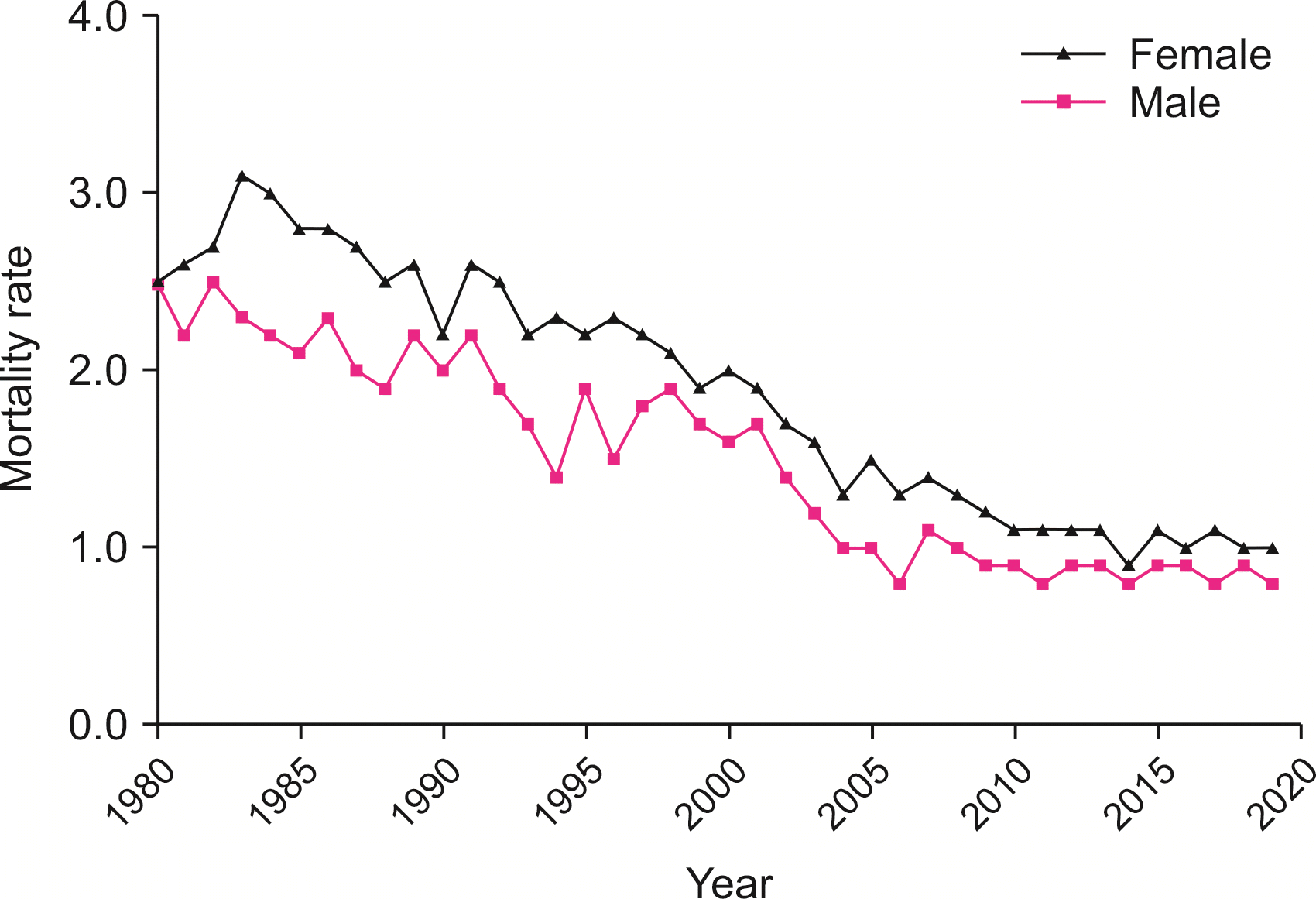
Table 1
Joinpoint analysis of all Australian and sex-specific incidence rates
| GBC incidence group | Perioda) | Years | APC | 95% CI | Significance (p-value)b) |
|---|---|---|---|---|---|
| All Australians | 1 | 1982–1996 | 0.10% | –0.50 to 0.70 | NS (0.84) |
| 2 | 1996–2005 | –2.80% | –4.30 to –1.20 | S (< 0.01) | |
| 3 | 2005–2017 | 2.20% | 1.40 to 3.10 | S (< 0.01) | |
| Male | 1 | 1982–1997 | 0.70% | –0.20 to 1.60 | NS (0.14) |
| 2 | 1997–2003 | –4.80% | –9.70 to 0.50 | NS (0.07) | |
| 3 | 2003–2017 | 2.60% | 1.60 to 3.70 | S (< 0.01) | |
| Female | 1 | 1982–1998 | –0.40% | –0.90 to 0.10 | NS (0.11) |
| 2 | 1998–2005 | –3.20% | –5.50 to –0.90 | S (< 0.01) | |
| 3 | 2005–2017 | 1.70% | 0.80 to 2.50 | S (< 0.01) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download