Abstract
Backgrounds/Aims
Middle hepatic vein (MHV) is usually preserved as a part of the right or left hepatectomy in order preserve the venous outflow of remnant liver. The aim of this study was to evaluate if resection of MHV could influence post-resection outcomes of standard right or left hepatectomy.
Methods
Patients who underwent standard right or left hepatectomy between January 2015 and December 2019 were included. Anatomical remnant liver volumes were measured retrospectively using the Hermes workstation (Hermes Medical Solutions AB, Stockholm, Sweden). Uni- and multi-variate analyses were performed to assess the difference in outcomes of those with preservation of MHV and those without preservation.
Results
A total of 144 patients were included. Right hepatectomy was performed for 114 (79.2%) and left hepatectomy was performed for 30 (20.8%) patients. MHV was resected for 13 (9.0%) in addition to the standard right or left hepatectomy. Median remnant liver volume was significantly higher in the MHV resected group (p < 0.01). There was no significant difference in serum level of bilirubin, international normalized ratio, alanine aminotransferase, creatinine on postoperative day 1, 3, 5, or 10, ≥ grade IIIa complications (p = 0.44), or 90-day mortality (p = 0.41). On multivariable analysis, resection of the MHV did not influence the incidence of post hepatectomy liver failure (p = 0.52).
Standard right or left hepatectomy extends up to the middle hepatic vein (MHV) without including the MHV. However, when the tumour is in proximity to MHV, MHV needs to be sacrificed as part of surgical resection. Venous drainage of segment 5/8 and segment 4 is usually based on MHV. Loss of MHV can cause venous congestion and ischemia of S4 or S5/8 depending on the type of resection, resulting in an increased risk of postoperative complications. The risk of post hepatectomy liver failure (PHLF) after major resections ranges from 1.2% to 30%. Treatment options are limited, with mortality rate as high as 20% in patients with severe PHLF [1]. Adequate future liver remnant volume (FLRV) is an important factor in mitigating the development of PHLF [2]. Preoperative anatomical volumetry is considered a measure of adequate remnant volumes. However, the calculation of preoperative volumes does not assess the extent of congestion of liver segments or collateral damage due to resection of MHV.
One of the phenomena leading to PHLF in cases where the MHV is resected is the development of portal hypertension since the venous flow is redirected as the portal vein works as a refluxing vein [3]. Such an increase in portal pressure can cause endothelial damage and reduce liver regeneration, ultimately resulting in small-for-size syndrome. For this reason, the donor MHV is preserved and the graft MHV is always reconstructed in living donor liver transplant programs [4]. In contrast, studies from liver resection cohorts have reported that preserving MHV does not play an important role because of pre-existing intrahepatic venous collaterals in those with tumour residing on the MHV. There is no consensus regarding preservation of MHV or its relation with post hepatectomy outcomes [5]. Thus, the primary aim of this study was to evaluate where there might be a difference in post hepatectomy outcomes following major hepatectomy with or without preserving MHV.
A retrospective analysis was carried out using a prospectively maintained database at Queen Elizabeth Hospital, Birmingham, UK. A total of 144 patients underwent right or left hepatectomy between January 2015 and March 2019. Extended liver resections and minor resections were excluded.
Anatomical remnant liver volumes were measured retrospectively for all patients based on the last contrast-enhanced CT performed prior to surgery as part of a routine assessment. The platform used was the Hermes Hybrid Recon package (Hermes Medical Solutions, Stockholm, Sweden). Arterial, portal, and venous phases series were used for the volumetry. A standardized future liver remnant (FLR) was measured and expressed as the ratio of remnant liver volume to the standardized liver volume based on body surface area using formulas introduced by Vauthey et al. [6] and Urata et al. [7].
Volumes of liver resection were retrospectively assessed using Hermes software according to the operative procedure performed. Volumetry results of Hermes software were validated by correlating with dry weight of the specimen measured in the histopathology laboratory and retrieved from reports. One gram of parenchyma was considered equal to 1 cc or 1 mL liver volume on the volumetry.
Low central venous pressure anaesthesia was used routinely during parenchymal division. The Pringle manoeuvre was performed at the discretion of the operating surgeon. Energy devices (Cavitron Ultrasonic Surgical Apirator, CUSA; Integra, Ireland; Lotus Ultrasonic energy device; BOWA-electronic GmbH, Gomaringen, Germany; Thunderbeat; Olympus, Hamburg, Germany) were used according to the operative surgeon’s preference. It was the departmental policy for patients who had a prior chemotherapy to wait for at least six weeks between the completion of chemotherapy and liver resection surgery. Enhanced recovery pathway was followed in the later cohort.
The International Study Group of Liver surgery definition was used to identify patients with PHLF [1].
Data are expressed as mean ± standard deviation. Differences between groups were assessed by the chi-squared test and the Fisher’s exact test for categorical data. Continuous variables were analysed using Student’s t-test or Mann–Whitney U-test. Pearson’s correlation coefficient (r) was calculated to determine correlations. The predictive value was assessed by calculating the area under the receiver operator ROC curve (AUC). Variables showing significance in univariate analysis were entered into the multivariate logistic regression analysis in a backward elimination manner. Statistical analysis was performed using IBM SPSS 20.0 (IBM Corp., Armonk, NY, USA). A p-value < 0.05 was considered statistically significant.
A total of 144 patients and their computed tomography (CT) scans were evaluated retrospectively. The mean age was 65 years. There were 95 (65.9%) males. Indications for major hepatectomy were colorectal liver metastasis (67.0%), HCC (11.8%), cholangiocarcinoma (9.0%), and other types of metastases (13.2%). Right hepatectomy was performed for 114 (79.2%) patients and left hepatectomy was performed for 30 (20.8%) patients. MHV was resected for 13 (9.0%) patients, including 10 (76.9%) patients who underwent left hepatectomy and 3 (23.1%) patients who underwent right hepatectomy (Table 1).
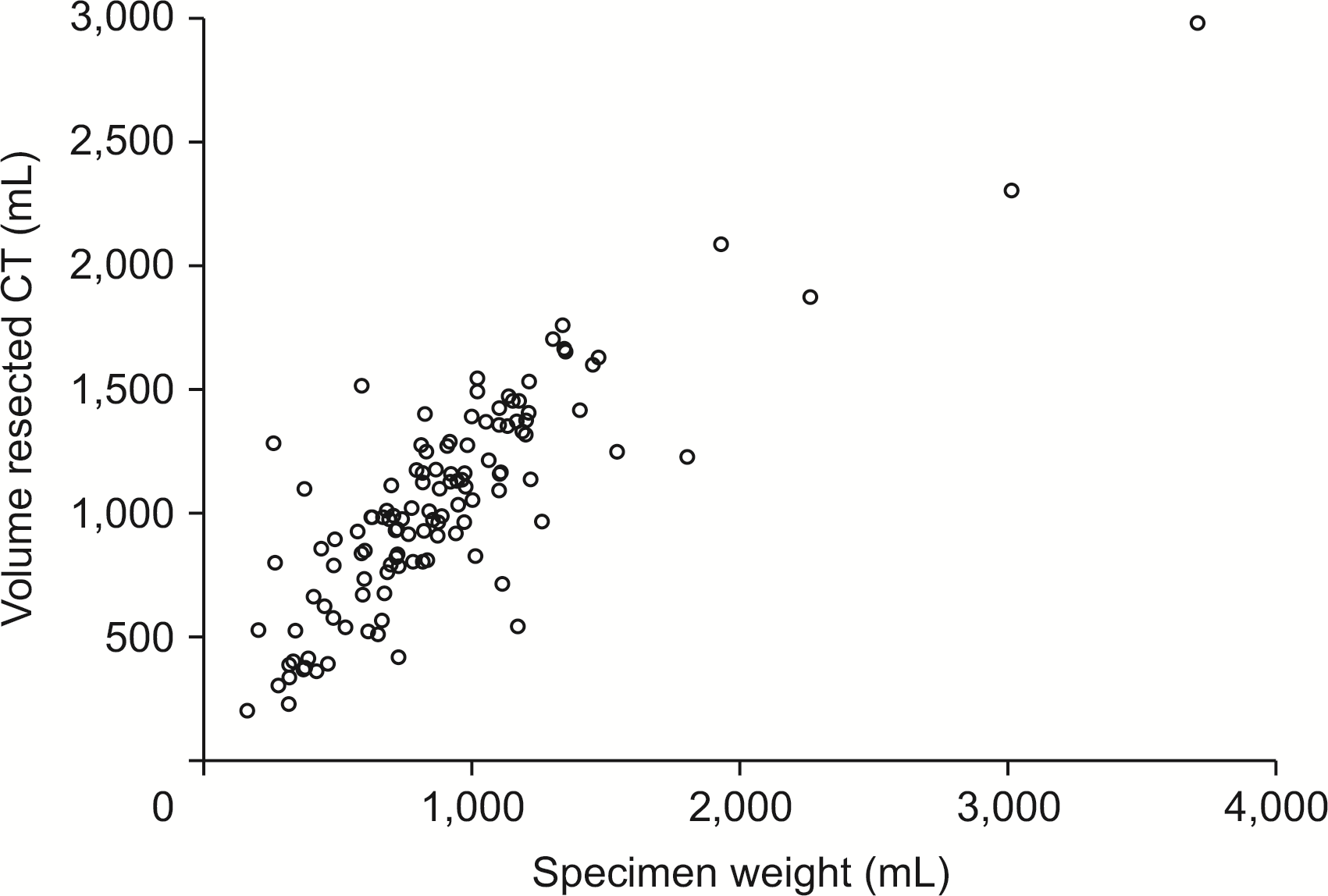
The mean total liver volume calculated with the Hermes Software was 1,688.41 ± 582.86 mL. The mean remnant volume was 517.31 ± 390.22 mL. The mean specimen weight was 880.4 ± 469.38 mL. The predicted volume resected that was calculated with the Hermes software was significantly correlated with the weight of the specimen (Pearson correlation = 0.8) (Fig. 1).
Remnant volumes: The remnant volume was 495 mL (181–2,030 mL) in the MHV preserved group and 1,077 mL (500–2,637 mL) in the MHV resected group. The standardised FLR was 0.31 (0.11–0.96) in the MHV preserved group and 0.72 (0.29–1.22) in the resected group, showing a significant (p < 0.01) difference between the two (Table 2).
Biochemical parameters: Median serum bilirubin levels (µmol/L) on postoperative day (POD) 1 (31 vs. 36 µmol/L), POD3 (27 vs. 20 µmol/L), and POD5 (28 vs. 16 µmol/L) were not significantly different between the MHV preserved group and the MHV resected groups (p = 0.56, p = 0.82, and p = 0.11, respectively). Median INR levels on POD1 (1 in both groups), POD3 (1.5 vs. 1.4), and POD5 (1.2 vs. 1.1) were not significantly different between the two groups. There were no significant differences in biochemical parameters evaluated between the two groups on postoperative day 1, 3, or 5 (Table 1). Median alanine aminotransferase levels (U/L) on POD1 (283 vs. 263), POD3 (173 vs. 145), and POD5 (114 vs. 111) were not significantly different between the two groups either (p = 0.99, p = 0.80, and p = 0.98, respectively) (Table 3).
Post hepatectomy liver failure: Overall incidence of PHLF was 19.4% (grade A: 9.8%; grade B: 6.2%; grade C: 3.4%). The incidence of PHLF was 19.8% in the MHV preserved group and 15.3% in MHV resected group (p = 0.52) (Table 1). In the multivariable analysis, resection of the MHV did not influence the PHLF rate. Presence of background steatohepatitis (hazard ratio [HR]: 11.592, 95% confidence interval [CI]: 4.125–32.57, p < 0.01) and FRLV less than 470 mL (HR: 14.528, 95% CI: 2.549–82.278, p < 0.01) were significantly associated with PHLF (Table 4). An standardised remnant liver volume with the Vauthey formula less than 27% was associated with PHLF in the univariate analysis, but not in the multivariate analysis. To avoid multicollinearity with various forms of volumes calculated, multivariate analyses was redone using only FRLV (Supplementary Table 1). It was found that resection of MHV did not influence the PHLF rate (p = 0.44).
Other postoperative outcomes: No differences were seen in postoperative complications (IIIa or above) (p = 0.44), postoperative hospital stay (10 days vs. 11 days, p = 0.87), or 90-day mortality rate (p = 0.41) between the two groups. The incidence of PHLF was not significant different between the two groups either (p = 0.52).
Adequate remnant parenchymal volume with appropriate arterial and portal inflow balanced by hepatic venous outflow is a major factor mitigating the risk of PHLF following liver resection. Sacrificing the MHV can result in an ischemic anterior segment or segment 4 of the liver due to venous congestion, which can lead to liver dysfunction and negatively influence post-surgical outcomes. The effect of middle hepatic venous resection in the liver has been studied exhaustively in the field of living donor liver transplantation [6]. Still, there are relatively fewer studies addressing the issue in patients requiring a liver resection. Reconstruction of the hepatic vein plays a critical role in preserving the venous drainage of the residual liver volume. It reduces the risk of small-for-size syndrome. Here, we report that resecting the MHV does not influence the incidence of PHLF or survival in patients undergoing liver resection with an adequate remnant liver volume.
The extent of liver resection directly influences morbidity after major hepatectomy. A remnant liver volume of 20% to 30% is considered safe in patients with normal background liver. A higher volume of over 40% is deemed safe in patients with an underlying structural disease [8]. In the current study, the median percentage of remnant liver volume was 27% in patients with PHLF and 42% in patients without PHLF. The remnant liver volume in patients with MHV preserved was 30% vs. 62% in those with MHV resected. The remnant liver volume was assessed using Hermes CT volumetry. Mevis, Synapse, Osirix, and Image J are commonly used semi-automatic software programmes available to evaluate liver volumetry. Hermes is one such platform mainly used in SPECT-CT studies [9]. The current study validated results of volumetric analysis by comparing the resected specimen volume with CT volumetry measurements. Pearson correlation was high at 0.84 (p < 0.01), proving the reliability of the software. It was also noted to be an easy-to-use, efficient software without inter-observer variability by the authors (AN, BD).
Despite having adequate volumetry assessments, anatomical volumetric studies do not consider pathophysiological changes such as venous congestion while assessing remnant volumes, the extent of ischemia (percent of the remnant liver), or resulting portal hypertension. Portal hypertension is due to reflux flow into the portal vein. Bogner et al. [10] have reported that post-resection portal venous pressure is significantly higher (5.0 ± 4.6 vs. 2.8 ± 5.3 mmHg, p = 0.04) in patients who have undergone a right hepatectomy with MHV resection. Such an increased portal flow contributes to endothelial damage, resulting in small for flow syndrome.
Inoue et al. [3] assessed the relationship between resection of MHV and liver regeneration by performing CT scans on day 7 as well as at 1, 2, 5, and 12 months after resection and reported that the regeneration rate is significantly higher when the MHV is preserved in cases undergoing a right hepatectomy. Other studies have evaluated liver volumes at 3, 6, and 12 months postoperatively and found that disruption of the MHV during a major hepatectomy does not impair liver function [11]. There can be several plausible explanations for such a difference in outcomes. One school of thought is that collateralisation has already begun to form before liver resection either as a result of continuous compression of the tumour in small branches that drain in the MHV or because of obstruction secondary to tumoral invasion [12]. Such collateralization also allows reversed portal flow from the area where no drainage exists towards areas where the drainage is kept intact via the intrahepatic portal vein [13].
In addition to the presence and size of collaterals, the percentage volume of the congested liver of the remnant liver remains an important factor influencing outcomes. Sano et al. [14] recommend reconstruction of the hepatic vein or its tributaries when the remaining liver volume is less than 30% of the standard liver volume in liver resection or less than 40% in a liver transplantation cohort. Mise et al. [15] recommend hepatic vein reconstruction in patients with non-congested liver remnant volume smaller than 40% of the total liver volume when Indocyanine green retention rate at 15 minutes is less than 10%, or if the non-congested liver remnant volume is smaller than 50% of the total liver volume when ICGR15 is 10%–20%. Anatomical variations such as the presence of a scissural vein is also associated with better regeneration of the remnant liver when MHV needs to be sacrificed.
The current study has limitations. This study does not facilitate a direct comparison of outcomes when MHV is sacrificed in patients with less than inadequate or borderline FLR, although such a comparison is not feasible for prospective evaluation. Another limitation is the retrospective nature of volume assessments. However, the present study captured clinical events from a large Western cohort undergoing major standard anatomical liver resection with or without MHV and found that sacrificing the MHV did not impact the incidence of PHLF in patients with adequate remnant liver volume.
Supplementary data related to this article can be found at https://doi.org/10.14701/ahbps.21-159.
ACKNOWLEDGEMENTS
The Preliminary findings were presented at the 14th Congress of the European-African Hepato-Pancreato-Biliary Association (E-AHPBA), 15–17 September 2021, Bibalo. HPB 2021;23(Suppl 3):801.
REFERENCES
1. Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. 2011; Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 149:713–724. DOI: 10.1016/j.surg.2010.10.001. PMID: 21236455.
2. Lee JW, Lee JH, Park Y, Lee W, Kwon J, Song KB, et al. 2020; Risk factors of posthepatectomy liver failure for perihilar cholangiocarcinoma: risk score and significance of future liver remnant volume-to-body weight ratio. J Surg Oncol. 122:469–479. DOI: 10.1002/jso.25974. PMID: 32424895.
3. Inoue Y, Suzuki Y, Ota M, Fujii K, Kawaguchi N, Shimizu T, et al. 2018; Comparison of regeneration of remnant liver after hemihepatectomy with or without the middle hepatic vein. World J Surg. 42:1100–1110. DOI: 10.1007/s00268-017-4225-y. PMID: 28929234.
4. Yu PF, Wu J, Zheng SS. 2007; Management of the middle hepatic vein and its tributaries in right lobe living donor liver transplantation. Hepatobiliary Pancreat Dis Int. 6:358–363. PMID: 17690029.
5. Varghese CT, Bharathan VK, Gopalakrishnan U, Balakrishnan D, Menon RN, Sudheer OV, et al. 2018; Randomized trial on extended versus modified right lobe grafts in living donor liver transplantation. Liver Transpl. 24:888–896. DOI: 10.1002/lt.25014. PMID: 29350831.
6. Vauthey JN, Chaoui A, Do KA, Bilimoria MM, Fenstermacher MJ, Charnsangavej C, et al. 2000; Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 127:512–519. DOI: 10.1067/msy.2000.105294. PMID: 10819059.
7. Urata K, Kawasaki S, Matsunami H, Hashikura Y, Ikegami T, Ishizone S, et al. 1995; Calculation of child and adult standard liver volume for liver transplantation. Hepatology. 21:1317–1321. DOI: 10.1002/hep.1840210515. PMID: 7737637.
8. Guglielmi A, Ruzzenente A, Conci S, Valdegamberi A, Iacono C. 2012; How much remnant is enough in liver resection? Dig Surg. 29:6–17. DOI: 10.1159/000335713. PMID: 22441614.
9. Dasari BVM, Wilson M, Pufal K, Kadam P, Hodson J, Roberts KJ, et al. 2021; Variations between the anatomical and functional distribution, based on 99m technetium -mebrofinate SPECT-CT scan, in patients at risk of post hepatectomy liver failure. HPB (Oxford). 23:1807–1814. DOI: 10.1016/j.hpb.2021.04.014. PMID: 33975803.
10. Bogner A, Reissfelder C, Striebel F, Mehrabi A, Ghamarnejad O, Rahbari M, et al. 2021; Intraoperative increase of portal venous pressure is an immediate predictor of posthepatectomy liver failure after major hepatectomy: a prospective study. Ann Surg. 274:e10–e17. DOI: 10.1097/SLA.0000000000003496. PMID: 31356261.
11. Faitot F, Vibert E, Salloum C, Gorden DL, Coscas F, Adam R, et al. 2012; Importance of conserving middle hepatic vein distal branches for homogeneous regeneration of the left liver after right hepatectomy. HPB (Oxford). 14:746–753. DOI: 10.1111/j.1477-2574.2012.00514.x. PMID: 23043663. PMCID: PMC3482670.
12. Morioka D, Tanaka K, Sekido H, Matsuo K, Sugita M, Ueda M, et al. 2006; Disruption of the middle hepatic vein is not crucial for liver regeneration of the remnant liver after right hemihepatectomy for hepatic tumors. Ann Surg Oncol. 13:1560–1568. DOI: 10.1245/s10434-006-9087-8. PMID: 17024557.
13. Scatton O, Plasse M, Dondero F, Vilgrain V, Sauvanet A, Belghiti J. 2008; Impact of localized congestion related to venous deprivation after hepatectomy. Surgery. 143:483–489. DOI: 10.1016/j.surg.2007.11.002. PMID: 18374045.
14. Sano T, Shimada K, Nara S, Sakamoto Y, Kosuge T. 2008; Reconstruction of hepatic venous tributaries using a Y-shaped left portal vein graft harvested from a resected left liver. Hepatogastroenterology. 55:228–230. PMID: 18507112.
15. Mise Y, Hasegawa K, Satou S, Aoki T, Beck Y, Sugawara Y, et al. 2011; Venous reconstruction based on virtual liver resection to avoid congestion in the liver remnant. Br J Surg. 98:1742–1751. DOI: 10.1002/bjs.7670. PMID: 22034181.
Table 1
Demographic characteristics
Table 2
Volumetry values in middle hepatic vein (MHV) preserved and MHV resected groups
Table 3
Biochemical parameters including preoperative values in middle hepatic vein (MHV) preserved and MHV resected groups
Table 4
Multivariable analysis of risk factors for PHLF
| Factor | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
|
|
|
||||
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age | 0.983 (0.949–1.019) | 0.35 | |||
| BMI | 1.059 (0.957–1.171) | 0.27 | |||
| Male sex | 8.855 (2.005–39.104) | < 0.01 | |||
| Diabetes | 0.960 (0.294–3.133) | 0.95 | |||
| COPD | 0.270 (0.068–1.082) | 0.06 | 14.707 (1.750–123.602) | 0.01* | |
| Background steatohepatitis | 11.701 (4.164–32.875) | < 0.01* | 11.592 (4.125–32.57) | < 0.01* | |
| RLV (Hermes) | 1.004 (1.001–1.007) | < 0.01* | |||
| RLV/Bw | 1.397 (1.123–1.737) | < 0.01* | |||
| RLV/LV(< 28% vs. > 28%) | 4.828 (1.836–12.697) | < 0.01* | |||
| sRLV (Vauthey) (< 27% vs. > 27%) | 5.461 (2.032–14.767) | < 0.01* | |||
| Bilirubin day 1 (< 38 vs. > 38) | 10.054 (3.728–27.110) | < 0.01* | 29.849 (5.112–174.299) | < 0.01* | |
| MHV resection | 2.263 (0.290–17.640) | 0.44 | |||




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download