Abstract
Backgrounds/Aims
Postoperative pain management is a key to enhanced recovery after surgery. The aim of this study was to evaluate clinical effect of preoperative intravenous (IV) non-steroidal anti-inflammatory drugs (NSAIDs) on relief of postoperative pain in patients after laparoscopic cholecystectomy.
Methods
This single center, retrospective study was conducted between September 2019 and May 2020. A total of 163 patients were divided into two groups: Ibuprofen group (preoperative IV ibuprofen, n = 77) and Ketorolac group (preoperative IV ketorolac, n = 86). The primary outcome was postoperative pain score measured immediately in the recovery room.
Results
There was no difference in demographic characteristics between the two groups of patients. Postoperative pain score measured immediately in the recovery room was significantly higher in the Ibuprofen group than in the Ketorolac group (mean value: 5.09 vs. 4.61; p = 0.027). The number of patients who needed analgesics immediately in the recovery room was also higher in the Ibuprofen group than in the Ketorolac group (28 [36.4%] vs. 18 [20.9%]; p = 0.036).
Conclusions
In this study, preoperative IV injection with ketorolac reduced postoperative pain and analgesic requirement in the recovery room more effectively than that with ibuprofen. However, both showed similar effects on peak pain and pain at discharge. Numbers of patients requiring additional analgesics were also similar between the two groups.
Postoperative pain management is a key to enhanced recovery after surgery (ERAS). As inadequate relief of postoperative pain can result in prolonged admission or readmission [1-3], optimal pain management using multimodal analgesic agents should be provided for patients during the perioperative period.
Non-steroidal anti-inflammatory drugs (NSAIDs) have been used in various clinical settings to reduce pain and inflammatory reactions [4]. NSAIDs in combination with opioid analgesics show more effective relief of pain than using NSAIDs alone [5-7]. Recently, due to undesirable adverse effects of opioids such as nausea, unexpected sedation, respiratory depression, and subsequent addiction [8-14], opioids are recommended to be reserved for rescue analgesia only15. Through administration of NSAIDs, opioid-sparing effects are in the range of 20%–30% [15-19].
Laparoscopic cholecystectomy is the mainstay treatment for symptomatic gallstones and benign biliary diseases. Despite its minimal invasiveness with minor tissue trauma, acute postoperative pain has remained a clinical challenge [20]. Therefore, the PROSPECT (Procedure Specific Postoperative Pain Management) Working Group has recommended basic multimodal analgesic techniques including paracetamol and NSAIDs or cyclooxygenase-2 (COX-2) inhibitors based on randomized controlled trials [21]. As preoperative initiation of NSAIDs could decrease both postoperative pain and analgesic requirement [22-28], Working group recommended to start paracetamol and NSAIDs before or during operation.
In this study, we compared two NSAIDs (Ibuprofen vs. Ketolorac) approved for intravenous (IV) drug use by the U.S. Food and Drug Administration (FDA) to determine which one would be more effective for postoperative pain relief in patients after laparoscopic cholecystectomy.
This single center, retrospective study was conducted between September 2019 and May 2020. Patients aged over 18 years who were admitted to the hospital due to acute or chronic cholecystitis, gallbladder (GB) stones or polyps, or suspicious GB cancer in the study period were enrolled. Patients received preoperative percutaneous cholecystostomy. Those who were diagnosed with Grade II or III acute cholecystitis and those who received common bile duct exploration or open conversion were excluded from this study. All patients underwent laparoscopic cholecystectomy under general anesthesia by one of three hepatobiliary surgeons. Of 205 patients, 163 with complete medical records were eligible for this study. Demographic data including age, sex, laboratory findings, surgical procedures, intraoperative events, and postoperative pain score were reviewed.
Among 163 patients, 77 received ibuprofen (Ibuprofen group) and 86 received ketorolac tromethamine (Ketorolac group) pre- and postoperatively. The selection of drugs was based on each surgeon’s preference. Before induction of general anesthesia, single dose of ibuprofen (Amoburofen 400 mg) or ketorolac tromethamine (Ketorac 30 mg) was injected intravenously with 100 mL of 5% dextrose. During anesthesia, the use and dosage of analgesics were determined according to the judgment of the anesthesiologist. Remifentanil was used in all patients due to its sedative effect and fentanyl was used as an analgesic. For initial pain control after operation, ibuprofen or ketorolac was used according to preoperative injection. All patients could receive additional opioids (tramadol 50 mg) by intramuscular (IM) or IV administration if needed for breakthrough pain even after ibuprofen or ketorolac injection. However, there was no routine IM or IV analgesic use after initial pain control. Tramadol was used when the patient wanted additional IV analgesics before discharge. Additional IV Ibuprofen or ketorolac was used following preoperative injection only if the patient desired, even after additional tramadol was administered to the patient. Additional IV analgesics were used with an interval of at least four hours. Oral aceclofenac 100 mg was initiated twice a day from postoperative day #1. It was continued until discharge (Fig. 1). Patient-controlled analgesia was not placed in this study.
Postoperative pain of each patient was measured immediately in the recovery room and monitored every eight hours using the visual analogue scale (VAS). Additional VAS score was recorded before additional analgesic usage. The VAS allowed patients to rate their pain on a scale of 0 (without pain) to 10 (with severe pain). The primary outcome was postoperative pain score measured immediately in the recovery room. The number of patients who needed analgesics right after arrival to the recovery room, the peak of postoperative pain score, the pain score at discharge, and the total number of administration of additional opioids in the postoperative period were reviewed based on electronic medical record.
All statistical analyses were performed using IBM SPSS software, ver. 25.0 (IBM Corp., Armonk, NY, USA). Categorical variables were analyzed with either chi-squared test or Fisher’s exact test, while continuous variables were analyzed with Student’s t-test. Statistical significance was considered when p-value was less than 0.05.
There were no significant differences in baseline characteristics such as the number of patients with abdominal pain, preoperative pain score assessed by the VAS, surgical procedures, or postoperative clinical outcomes between the two groups. The percentage of emergency surgery did not differ significantly either between the two groups (Ibuprofen vs. Ketorolac = 10.4% vs. 12.8%; p = 0.808). Estimated blood loss during surgery was minimal in both groups. The mean length of postoperative hospital stay was 1.4 days in both groups without showing significant difference. There was no postoperative complication in either group (Table 1).
Postoperative pain score measured immediately in the recovery room was significantly lower in the Ketorolac group than in the Ibuprofen group (Ibuprofen group 5.09 ± 1.45, Ketorolac group 4.61 ± 1.23, p = 0.027) (Fig. 2). The number of patients who needed analgesics immediately in the recovery room was also higher in the Ibuprofen group than in the Ketorolac group (Ibuprofen group 28 [36.4%], Ketorolac group 18 [20.9%], p = 0.036). However, there were no significant differences in the ratio (p > 0.999) or dosage (p = 0.786) of analgesic use during anesthesia between the two groups. Peak postoperative pain score during hospitalization (p = 0.113), pain score on the day of discharge (p = 0.320), and total number of administration of additional opioids in the postoperative period (p = 0.770) were not significantly different between the two groups (Table 2).
This study demonstrated that preoperative IV injection with ketorolac reduced postoperative pain and analgesic requirement at recovery room more effectively than that with ibuprofen. However, they showed similar effects on the peak pain and pain at discharge. The two groups also showed similar total numbers of patients needing additional opioids during the postoperative period.
Traditional opioid-dependent pain management is getting unpopular due to surgeon’s and patient’s concern for side effects such as delayed discharge and/or unexpected addiction [29]. For this reason, NSAIDs are attractive alternatives. They also could be used in combination with opioids. Therefore, NSAIDs are regarded as a key component of multimodal regimens for postoperative analgesia [30].
The mechanism of action of NSAIDs is modulation of local inflammatory response by inhibiting prostaglandin synthesis. COX-1 is known to generate prostaglandins involved in protection of gastrointestinal mucosa, while COX-2 is induced at sites of inflammation that generate prostaglandins for mediating inflammation and pain. Therefore, anti-inflammatory and analgesic effects of NSAIDs seem to be mainly mediated by inhibition of COX-2, whereas inhibition of COX-1 has a negative effect on the gastrointestinal mucosa, resulting in severe morbidity after using NSAIDs [31-33]. IV ketorolac has been used approved by the FDA in the United States to treat pain. It was the only IV NSAID until ibuprofen was approved for IV use in 2009. Ketorolac can inhibit both COX-1 and COX-2 with a ratio of 330 : 1 [34]. This composition contributes to secondary effect on gastrointestinal mucosal bleeding or operative site bleeding in the perioperative period [35-37]. However, a recent meta-analysis was unable to correlate ketorolac administration and bleeding risk because the number of bleeding events was too low [38]. In another meta-analysis of randomized controlled trials, ketorolac did not increase postoperative bleeding in various surgical procedures [39].
Ibuprofen is also a non-selective inhibitor of both COX-1 and COX-2 with a ratio of 2.5 : 1. It has a reduced risk of bleeding or problems of gastrointestinal mucosa compared to ketorolac [34,40]. A recent meta-analysis has demonstrated that preoperative single dose of IV ibuprofen could reduce pain and opioid consumption until postoperative 24 hours. Kim et al. [41] have reported results based on six studies comparing IV ibuprofen with placebo in patients undergoing surgeries including laparoscopic cholecystectomy, septorhinoplasty, thyroidectomy, and pancreaticoduodenectomy.
In the present study, we aimed to evaluate which type of IV NSAID (ibuprofen or ketorolac) would be more effective in reducing postoperative pain scores and the need for additional analgesics after laparoscopic cholecystectomy. This study was designed to select the type of NSAIDs not on the basis of randomization, but according to the surgeon’s preference. Another limitation of this study was that we evaluated postoperative pain score until the discharge from the hospital. We did not follow patients to determine whether they finally had a long-term chronic pain or not. Another limitation of this study was that we did not analyze different doses of IV ibuprofen. IV Ibuprofen is available in 400 to 800 mg dosage for a single dose. However, we only analyzed 400 mg ibuprofen in this study because of surgeons’ preference. No patients received 800 mg IV ibuprofen for a single dose.
The current study is noteworthy in that it shows comparable effects of two types of NSAIDs irrespective of inhibition rate of COX-1 to COX-2. In this study, there was no bleeding event of operative site or gastrointestinal mucosa in either group. Therefore, ketorolac and ibuprofen can both be used in preoperative settings with safety for patients undergoing laparoscopic cholecystectomy.
In conclusion, preoperative IV ketorolac reduced postoperative pain score and analgesic requirement in the recovery room more effectively than preoperative IV ibuprofen in patients undergoing laparoscopic cholecystectomy. Further prospective randomized controlled trials are needed to give us more conclusive evidence for the use of preoperative IV NSAIDs.
REFERENCES
1. van Boekel RLM, Warlé MC, Nielen RGC, Vissers KCP, van der Sande R, Bronkhorst EM, et al. 2019; Relationship between postoperative pain and overall 30-day complications in a broad surgical population: an observational study. Ann Surg. 269:856–865. DOI: 10.1097/SLA.0000000000002583. PMID: 29135493.
2. Rosero EB, Joshi GP. 2017; Hospital readmission after ambulatory laparoscopic cholecystectomy: incidence and predictors. J Surg Res. 219:108–115. DOI: 10.1016/j.jss.2017.05.071. PMID: 29078868.
3. Glare P, Aubrey KR, Myles PS. 2019; Transition from acute to chronic pain after surgery. Lancet. 393:1537–1546. DOI: 10.1016/S0140-6736(19)30352-6. PMID: 30983589.
4. Gupta A, Bah M. 2016; NSAIDs in the treatment of postoperative pain. Curr Pain Headache Rep. 20:62. DOI: 10.1007/s11916-016-0591-7. PMID: 27841015.
5. Raffa RB. 2001; Pharmacology of oral combination analgesics: rational therapy for pain. J Clin Pharm Ther. 26:257–264. DOI: 10.1046/j.1365-2710.2001.00355.x. PMID: 11493367.
6. McQuay HJ, Moore RA, Berta A, Gainutdinovs O, Fülesdi B, Porvaneckas N, et al. 2016; Randomized clinical trial of dexketoprofen/tramadol 25 mg/75 mg in moderate-to-severe pain after total hip arthroplasty. Br J Anaesth. 116:269–276. DOI: 10.1093/bja/aev457. PMID: 26787797. PMCID: PMC4718147.
7. Moore RA, McQuay HJ, Tomaszewski J, Raba G, Tutunaru D, Lietuviete N, et al. 2016; Dexketoprofen/tramadol 25 mg/75 mg: randomised double-blind trial in moderate-to-severe acute pain after abdominal hysterectomy. BMC Anesthesiol. 16:9. DOI: 10.1186/s12871-016-0174-5. PMID: 26801905. PMCID: PMC4724087.
8. Kroll PB, Meadows L, Rock A, Pavliv L. 2011; A multicenter, randomized, double-blind, placebo-controlled trial of intravenous ibuprofen (i.v.-ibuprofen) in the management of postoperative pain following abdominal hysterectomy. Pain Pract. 11:23–32. DOI: 10.1111/j.1533-2500.2010.00402.x. PMID: 20642488.
9. Bookstaver PB, Miller AD, Rudisill CN, Norris LB. 2010; Intravenous ibuprofen: the first injectable product for the treatment of pain and fever. J Pain Res. 3:67–79. DOI: 10.2147/JPR.S6993. PMID: 21197311. PMCID: PMC3004645.
10. Singla N, Rock A, Pavliv L. 2010; A multi-center, randomized, double-blind placebo-controlled trial of intravenous-ibuprofen (IV-ibuprofen) for treatment of pain in post-operative orthopedic adult patients. Pain Med. 11:1284–1293. DOI: 10.1111/j.1526-4637.2010.00896.x. PMID: 20609131. PMCID: PMC2955972.
11. Moss JR, Watcha MF, Bendel LP, McCarthy DL, Witham SL, Glover CD. 2014; A multicenter, randomized, double-blind placebo-controlled, single dose trial of the safety and efficacy of intravenous ibuprofen for treatment of pain in pediatric patients undergoing tonsillectomy. Paediatr Anaesth. 24:483–489. DOI: 10.1111/pan.12381. PMID: 24646068.
12. Koh W, Nguyen KP, Jahr JS. 2015; Intravenous non-opioid analgesia for peri-and postoperative pain management: a scientific review of intravenous acetaminophen and ibuprofen. Korean J Anesthesiol. 68:3–12. DOI: 10.4097/kjae.2015.68.1.3. PMID: 25664148. PMCID: PMC4318862.
13. Holdgate A, Pollock T. 2004; Nonsteroidal anti-inflammatory drugs (NSAIDs) versus opioids for acute renal colic. Cochrane Database Syst Rev. (1):CD004137. DOI: 10.1002/14651858.CD004137.pub2. PMID: 15846699. PMCID: PMC6986698.
14. Beck DE, Margolin DA, Babin SF, Russo CT. 2015; Benefits of a multimodal regimen for postsurgical pain management in colorectal surgery. Ochsner J. 15:408–412. DOI: 10.1016/j.mpsur.2014.05.010. PMID: 26730224. PMCID: PMC4679301.
15. Joshi GP, Kehlet H. 2019; Postoperative pain management in the era of ERAS: an overview. Best Pract Res Clin Anaesthesiol. 33:259–267. DOI: 10.1016/j.bpa.2019.07.016. PMID: 31785712.
16. Horattas MC, Evans S, Sloan-Stakleff KD, Lee C, Snoke JW. 2004; Does preoperative rofecoxib (Vioxx) decrease postoperative pain with laparoscopic cholecystectomy? Am J Surg. 188:271–276. DOI: 10.1016/j.amjsurg.2004.06.007. PMID: 15450833.
17. Gan TJ, Joshi GP, Viscusi E, Cheung RY, Dodge W, Fort JG, et al. 2004; Preoperative parenteral parecoxib and follow-up oral valdecoxib reduce length of stay and improve quality of patient recovery after laparoscopic cholecystectomy surgery. Anesth Analg. 98:1665–1673. DOI: 10.1213/01.ANE.0000117001.44280.F3. PMID: 15155324.
18. Lane GE, Lathrop JC, Boysen DA, Lane RC. 1996; Effect of intramuscular intraoperative pain medication on narcotic usage after laparoscopic cholecystectomy. Am Surg. 62:907–910. PMID: 8895711.
19. Liu J, Ding Y, White PF, Feinstein R, Shear JM. 1993; Effects of ketorolac on postoperative analgesia and ventilatory function after laparoscopic cholecystectomy. Anesth Analg. 76:1061–1066. DOI: 10.1213/00000539-199305000-00026. PMID: 8484508.
20. Bisgaard T, Kehlet H, Rosenberg J. 2001; Pain and convalescence after laparoscopic cholecystectomy. Eur J Surg. 167:84–96. DOI: 10.1080/110241501750070510. PMID: 11266262.
21. Barazanchi AWH, MacFater WS, Rahiri JL, Tutone S, Hill AG, Joshi GP. 2018; Evidence-based management of pain after laparoscopic cholecystectomy: a PROSPECT review update. Br J Anaesth. 121:787–803. DOI: 10.1016/j.bja.2018.06.023. PMID: 30236241.
22. Puura A, Puolakka P, Rorarius M, Salmelin R, Lindgren L. 2006; Etoricoxib pre-medication for post-operative pain after laparoscopic cholecystectomy. Acta Anaesthesiol Scand. 50:688–693. DOI: 10.1111/j.1399-6576.2006.01049.x. PMID: 16987363.
23. Papadima A, Lagoudianakis EE, Antonakis PT, Pattas M, Kremastinou F, Katergiannakis V, et al. 2007; Parecoxib vs. lornoxicam in the treatment of postoperative pain after laparoscopic cholecystectomy: a prospective randomized placebo-controlled trial. Eur J Anaesthesiol. 24:154–158. DOI: 10.1017/S0265021506001293. PMID: 16938157.
24. Kocaayan E, Ozkardeşler S, Ozzeybek D, Bayindir S, Akan M. 2007; Comparison of effects of preoperatively administered lornoxicam and tenoxicam on morphine consumption after laparoscopic cholecystectomy. Eur J Anaesthesiol. 24:714–719. DOI: 10.1017/S0265021507000300. PMID: 17519049.
25. Sandhu T, Paiboonworachat S, Ko-iam W. 2011; Effects of preemptive analgesia in laparoscopic cholecystectomy: a double-blind randomized controlled trial. Surg Endosc. 25:23–27. DOI: 10.1007/s00464-010-1122-y. PMID: 20589515.
26. Shuying L, Xiao W, Peng L, Tao Z, Ziying L, Liang Z. 2014; Preoperative intravenous parecoxib reduces length of stay on ambulatory laparoscopic cholecystectomy. Int J Surg. 12:464–468. DOI: 10.1016/j.ijsu.2014.03.013. PMID: 24681179.
27. Gulcin Ural S, Yener O, Sahin H, Simsek T, Aydinli B, Ozgok A. 2014; The comparison of analgesic effects of various administration methods of diclofenac sodium, transdermal, oral and intramuscular, in early postoperative period in laparoscopic cholecystectomy operations. Pak J Med Sci. 30:96–100. DOI: 10.12669/pjms.301.4140. PMID: 24639839. PMCID: PMC3955550.
28. Ahiskalioglu EO, Ahiskalioglu A, Aydin P, Yayik AM, Temiz A. 2017; Effects of single-dose preemptive intravenous ibuprofen on postoperative opioid consumption and acute pain after laparoscopic cholecystectomy. Medicine (Baltimore). 96:e6200. DOI: 10.1097/MD.0000000000006200. PMID: 28225506. PMCID: PMC5569427.
29. Uribe AA, Arbona FL, Flanigan DC, Kaeding CC, Palettas M, Bergese SD. 2018; Comparing the efficacy of IV ibuprofen and ketorolac in the management of postoperative pain following arthroscopic knee surgery. A randomized double-blind active comparator pilot study. Front Surg. 5:59. DOI: 10.3389/fsurg.2018.00059. PMID: 30338261. PMCID: PMC6178884.
30. Buvanendran A, Kroin JS. 2009; Multimodal analgesia for controlling acute postoperative pain. Curr Opin Anaesthesiol. 22:588–593. DOI: 10.1097/ACO.0b013e328330373a. PMID: 19606021.
31. Meade EA, Smith WL, DeWitt DL. 1993; Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 268:6610–6614. DOI: 10.1016/S0021-9258(18)53294-4. PMID: 8454631.
32. Fosslien E. 1998; Adverse effects of nonsteroidal anti-inflammatory drugs on the gastrointestinal system. Ann Clin Lab Sci. 28:67–81. PMID: 9558445.
33. Mitchell JA, Akarasereenont P, Thiemermann C, Flower RJ, Vane JR. 1993; Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci U S A. 90:11693–11697. DOI: 10.1073/pnas.90.24.11693. PMID: 8265610. PMCID: PMC48050.
34. Gago Martínez A, Escontrela Rodriguez B, Planas Roca A, Martínez Ruiz A. 2016; Intravenous ibuprofen for treatment of post-operative pain: a multicenter, double blind, placebo-controlled, randomized clinical trial. PLoS One. 11:e0154004. DOI: 10.1371/journal.pone.0154004. PMID: 27152748. PMCID: PMC4859493.
35. Singer AJ, Mynster CJ, McMahon BJ. 2003; The effect of IM ketorolac tromethamine on bleeding time: a prospective, interventional, controlled study. Am J Emerg Med. 21:441–443. DOI: 10.1016/S0735-6757(03)00100-1. PMID: 14523887.
36. Fragen RJ, Stulberg SD, Wixson R, Glisson S, Librojo E. 1995; Effect of ketorolac tromethamine on bleeding and on requirements for analgesia after total knee arthroplasty. J Bone Joint Surg Am. 77:998–1002. DOI: 10.2106/00004623-199507000-00004. PMID: 7608243.
37. Gabbott DA, Cohen AM, Mayor AH, Niemiro LA, Thomas TA. 1997; The influence of timing of ketorolac administration on post-operative analgesic requirements following total abdominal hysterectomy. Eur J Anaesthesiol. 14:610–615. DOI: 10.1097/00003643-199711000-00009. PMID: 9466097.
38. McNicol ED, Rowe E, Cooper TE. 2018; Ketorolac for postoperative pain in children. Cochrane Database Syst Rev. 7:CD012294. DOI: 10.1002/14651858.CD012294.pub2. PMID: 29981164. PMCID: PMC6513208.
39. Gobble RM, Hoang HLT, Kachniarz B, Orgill DP. 2014; Ketorolac does not increase perioperative bleeding: a meta-analysis of randomized controlled trials. Plast Reconstr Surg. 133:741–755. DOI: 10.1097/01.prs.0000438459.60474.b5. PMID: 24572864.
40. Orlando BJ, Lucido MJ, Malkowski MG. 2015; The structure of ibuprofen bound to cyclooxygenase-2. J Struct Biol. 189:62–66. DOI: 10.1016/j.jsb.2014.11.005. PMID: 25463020. PMCID: PMC4276492.
41. Kim SY, Lee S, Lee Y, Kim H, Kim KM. 2021; Effect of single dose preoperative intravenous ibuprofen on postoperative pain and opioid consumption: a systematic review and meta-analysis. Korean J Anesthesiol. 74:409–421. DOI: 10.4097/kja.21050. PMID: 33611881. PMCID: PMC8497907.
Fig. 1
Definition of ibuprofen group and ketorolac group. IVF, intravenous injection with fluid; IM, intramuscular; POD, postoperative day.
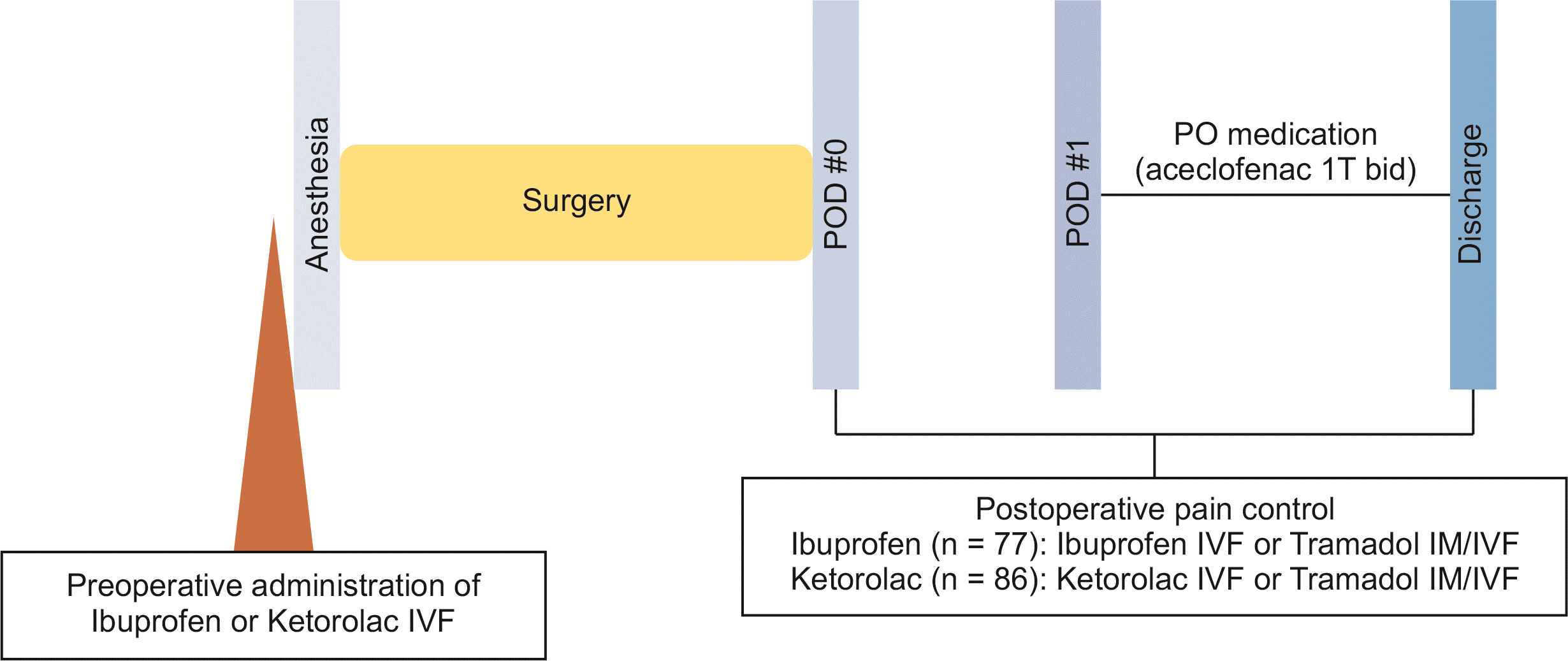
Fig. 2
Comparison of postoperative the visual analogue scale (VAS) score between the Ibuprofen group and the Ketorolac group.
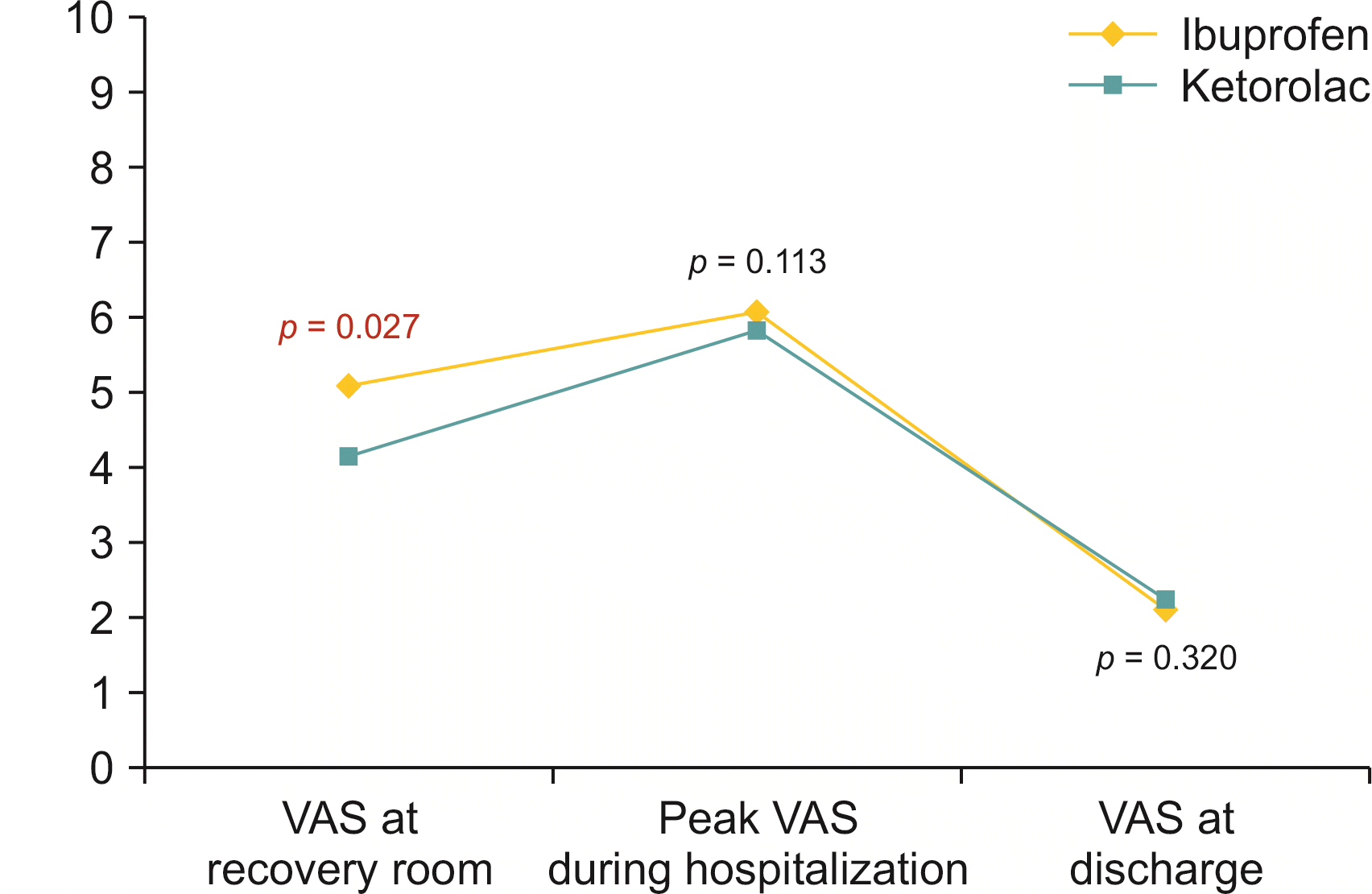
Table 1
Baseline characteristics and surgical procedures
Table 2
Comparison of analgesics and pain score using VAS between the ibuprofen group and the ketorolac group
| Variable | Ibuprofen group (n = 77) | Ketorolac group (n = 86) | p-value |
|---|---|---|---|
| Patients received analgesics during anesthesia | 46 (59.7) | 52 (60.5) | > 0.999 |
| Dosage of analgesics during anesthesia | 51.09 ± 7.37 | 51.54 ± 8.89 | 0.786 |
| Postoperative pain score at recovery room | 5.09 ± 1.45 | 4.61 ± 1.23 | 0.027* |
| No. of patients who need analgesics at recovery room | 28 (36.4) | 18 (20.9) | 0.036* |
| Pain score (peak) | 6.11 ± 1.07 | 5.82 ± 1.23 | 0.113 |
| Pain score (at discharge) | 2.11 ± 0.77 | 2.23 ± 0.87 | 0.320 |
| Total numbers of administration of additional anagesics during first 24 hours after surgery | 1.28 ± 1.04 | 1.23 ± 1.24 | 0.770 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download