Abstract
Backgrounds/Aims
Early recovery after surgery has become a popular trend. The aim of this study was to evaluate effect of nutritional intervention using Encover, an oral nutritional supplement, in patients undergoing hepato-biliary-pancreatic surgery.
Methods
This single center, prospective case-control study was conducted in Gangnam Severance Hospital from September 2018 to April 2019. Through randomization, patients were divided into an experimental group (30 patients) and a control group (30 patients). At postoperative seven days, the experimental group was instructed to take two packs of Encover (JW Pharmaceutical, Seoul, Korea) daily for seven days. Body cell mass index was measured at seven days after surgery and 14 days after discharge and Patient-Generated Subjective Global Assessment (PG-SGA) was performed at 14 days after discharge.
Results
Body cell mass index during outpatient follow-up was significantly decreased compared to that at discharge in both groups. However, the amount of body cell mass index showed no significant difference between postoperative seven days and outpatient follow- up in either group. During outpatient follow-up, the experimental group had a higher mean value of PG-SGA score than the control group (11.32 ± 3.46 vs. 9.48 ± 3.97; p = 0.037).
It has been reported that 20% to 50% of hospitalized patients are malnourished, with nutritional status worsening during hospitalization [1-4]. Poor dietary intake during hospitalization can cause deterioration of nutritional status. Malnutrition can increase complications including infection. It can also increase hospital stay and mortality [3,5,6]. Thus, appropriate nutritional therapy is needed. It is essential to point out areas to raise awareness for medical staff. In particular, in the case of gastrointestinal cancer, catabolism increases up to 10 days after surgery while protein anabolic activity decreases, which can result in loss of intestinal and skeletal muscle proteins in the body, postoperative weight loss, and cachexia, leading to decreased ability to recover for the body [4,7].
Recent surgical trends are focusing on early recovery after surgery. Enhanced Recovery After Surgery has been introduced. Early nutrition is strongly recommended for rapid recovery and proper nutrition of surgical patients [8-10]. However, when having a liquid to soft food diet after surgery, it is difficult to meet nutritional requirements due to the low caloric content per unit volume. After surgery, nutritional adequacy of a patient should be carefully reviewed due to problems such as indigestion, early postprandial fullness, bloating, and restriction of one-time meal intake [11,12]. Therefore, it is important to provide patients with a diet having proper amounts and adequate calories for sufficient nutrition to help their recovery.
Encover (JW Pharmaceutical, Seoul, Korea) is a product developed for patients who have difficulty in eating or lack nutritional intake [13,14]. It is an enteral nutrient that is used for tuberous nutrition, especially when oral nutrition is difficult for a long period of time. Patients who undergo major hepato-biliary-pancreatic surgery have a relatively low survival rate. Low body weight (body mass index [BMI] < 18.5 kg/m2) is directly related to the factors that increase the 5-year survival rate, which is less than 20% [15]. Intake of Encover is expected to improve body weight and biochemical/human measurements by contributing to continuous nutritional supplementation and increasing energy and protein intake. It is thought that it can increase survival rate by improving the quality of life of patients with liver and gallbladder disease.
The purpose of this study was to evaluate the effect of nutritional supplementation using Encover, an oral nutritional supplement, in patients undergoing major hepato-biliary-pancreatic surgery. Changes in weight, body fat, and muscle mass were determined after additional Encover was taken after surgery.
This was a single center, randomized case control study. It was conducted from September 2018 to April 2019. Patients in the Hepatobiliopancreatic Cancer Clinic, Gangnam Severance Hospital, Yonsei University College of Medicine were recruited. This study was approved by Gangnam Severance Institutional Review Board (approval number: 3-2017-0222). Written informed consent was obtained from all participants.
Inclusion criteria
• Patients scheduled for major hepato-biliary-pancreatic surgery
• Major hepato-biliary-pancreatic surgery: segmentectomy, hemihepatectomy, segmental resection of bile duct, radical cholecystectomy, distal pancratectomy, pancreatodudenectomy
Exclusion criteria
• Patient with poor adherence to oral nutritional supplements
• Liver failure or renal failure patients
• Patient unable to intake food orally
• Patient with severe ascites and edema affecting weight evaluation
• Patient whose cancer has metastasized to the brain
• Patient who have difficulty controlling blood sugar
• Patient with BMI > 30 kg/m2
• Illiteracy/foreign patient
• Patient with contraindications for Encover administration
The output of the sample size was based on an independent two-sample t-test. The expected ratio of maintaining muscle mass was determined by referring to a paper previously published by Kim et al. [16] Assuming an alpha value of 0.05 and 1-β (power) of 0.8, 22.271 samples would be needed for each group. Considering a dropout rate of 30%, 30 samples would be needed for each group.
All patients who were potential candidates for major hepato-biliary-pancreatic surgery were informed about this study. Only patients who voluntarily consented participated in this study. Patients who agreed to participate in this study were assigned to either a control group or an experimental group by randomization. Randomization was take place via an allocation randomization system before surgery. Patients were randomized to one of the two groups at a 1 : 1 ratio.
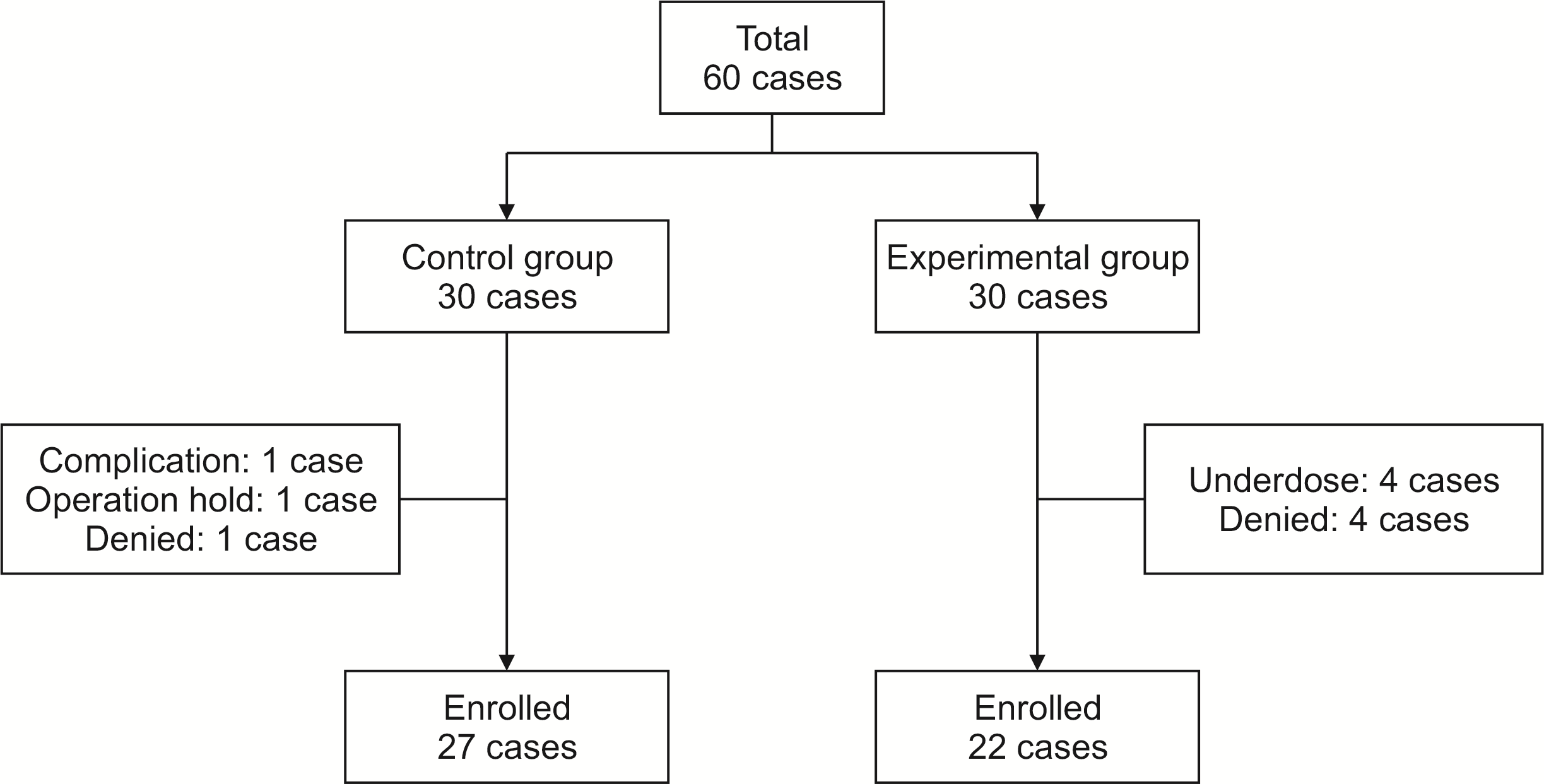
After patients were enrolled, their baseline characteristics were recorded. Patient-Generated Subjective Global Assessment (PG-SGA) (Fig. 1) was used to evaluate and record their current nutritional status. Body cell mass index measurements (Inbody S-10; Biospace, Seoul, Korea) were taken before surgery. At postoperative seven days, body cell mass index measurement was taken again. Patients were excluded from this study if they could not tolerate soft diet and/or refused to take Encover on postoperative seven days. At this time, the experimental group was instructed to take two packs of Encover daily for seven days while the control group was not instructed to take it. At 14 days after discharge, the last body cell mass index measurement was taken. PG-SGA was performed during outpatient follow-up. Patients taking less than 7 packs in total were excluded from this study. During the study period, a total of 8 cases were excluded from the Encover group as study denied or under dose in the experimental group. A total of 3 cases withdrew from this study due to operation hold, study denied, or complications in the control group (Fig. 2).
All statistical analyses were performed using IBM SPSS software, ver. 25.0 (IBM Corp., Armonk, NY, USA). Categorical variables were analyzed either by chi-squared test or Fisher’s exact test, while continuous variables were analyzed using Student’s t-test or Mann–Whitney U test. Statistical significance was considered when p-value was less than 0.05.
Clinicopathologic characteristics of patients are presented in Table 1. There was no significant difference in sex, age, or perioperative laboratory data related to nutritional status (such as albumin, prealbumin, cholesterol) between the control group and the experimental group. Major diagnosis, open and laparoscopic ratio, and main operation site (liver or pancreas) did not differ between the two groups either. Preoperative body cell mass index did not show significant difference between the two groups either (Table 2). During outpatient follow-up, body weight, body cell mass, soft lean mass, and fat free mass, but not fat mass, were significantly decreased than those at postoperative seven days in both groups (Table 3). When comparing the amount of change in body cell mass index from postoperative seven days to outpatient follow-up, there was no difference between the two groups. Body weight was decreased by 3.82 ± 2.84 kg in the control group and 4.27 ± 3.65 kg in the experimental group, showing no significant difference between the two (p = 0.627). There was no significant difference in the amount of change in body cell mass (p = 0.684), soft lean mass (p = 0.561), fat free mass (p = 0.578), or fat mass (p = 0.834) between the two groups either (Table 4).
When PG-SGA score and grade were compared, there was no difference in preoperative PG-SGA score or grade between the two groups. However, during postoperative outpatient follow-up, the experimental group had higher mean PG-SGA score (p = 0.037) and PG-SGA grade (p = 0.032) than the control group (Table 5).
This study was conducted in patients who underwent hepato-biliary-pancreatic surgery. The purpose of this study was to determine the effect of nutritional supplement using Encover by comparing postoperative body cell mass index following administration of additional oral nutritional supplements.
In this study, body cell mass index analysis was performed sing the multi-frequency impedance method with Inbody S-10. This method has been used in many studies as a simple and effective method to indirectly measure the body cell mass index [17-19]. PG-SGA is also widely used as a tool to evaluate the nutritional status of patients by examining changes in body weight, changes in meal intake, problems related to meals, and the level of physical activity through interviews with experienced nutritionists [20-22]. Nutritional risk screening 2002 method (NRS-2002) can also be used as a tool to evaluate nutritional status. However, PG-SGA is generally reported to have higher sensitivity than NRS-2002 [23,24].
Reduction of lean mass is associated with delayed wound healing, increased infection rate, increased morbidity, increased hospital stay, and increased medical costs for patients at risk of malnutrition such as surgical patients and critically ill patients [25]. It has been reported that reduction of lean mass has a strong correlation with increased mortality and organ failure [26]. In our study, most patients showed significant reductions in weight, body cell mass, lean mass, and muscle mass during outpatient follow-up compared to those before discharge.
The experimental group was given two packs of Encover per day for seven days. The total daily dose had 400 calories. The additional dose had 2,800 calories compared to the control group. Although each person’s food intake and digestibility might vary, one gram of protein is equivalent to about four kilocalories. Thus, simply adding one kilogram of fat requires 4,000 kilocalories. Although our study aimed to suppress decrease in body cell mass index through additional nutritional supplement, calories of additional nutritional supplement were limited.
Just as kidney patients need dialysis treatment and respiratory patients need ventilator, oral nutrition is an essential treatment for digestive disorder patients [27]. Taking Encover during the early recovery period after surgery for a short period of time is ineffective for diet weight gain. Other body cell mass indices (body cell mass, body fat, lean mass, muscle mass) were not significant changed either. Thus, it can be concluded that short-term effects of oral nutritional supplements could not affect body cell mass index. In addition, PG-SGA score and grade at outpatient follow-up were higher than those in the control group, indicating malnutrition in the experimental group.
Previous study have hypothesized that insufficient oral food intake is correlated with satiety and volume in the digestive tract [16]. Therefore, oral nutritional supplements such as high energy density in the diet are expected to help increase final caloric intake and subjective nutritional indicators after ingestion. However, PG-SGA score and grade showed opposite results. Taking Encover two packs a day might have reduced their original normal meal intake due to worsening of gastrointestinal satiety. This is a short-term side effect, suggesting that additional oral nutritional supplements may affect dietary intake. Additional oral nutritional supplements cannot be free from risks such as reduced intake of regular meals that can be better absorbed. In the future, in terms of nutritional management, when taking alternative nutrients, it is necessary to continuously monitor patient’s subjective nutritional status and closely monitor whether there is a possibility of digestive disorders or reduced intake.
This study has some limitations. First, it did not have a long-term follow-up after surgery. Interference aspects such as uncontrolled variables (gastrointestinal trouble) should also be considered. The effect on energy consumption rate according to the difference in exercise amount after each patient’s operation cannot be completely excluded.
In future studies, rather than using a short one-week dose period, we intend to investigate difference in body cell mass index according to the increase or decrease in dose by considering expansion and consumption of Encover as continuous variables.
In conclusion, short-term Encover doses after surgery may not produce significant results in weight gain or other body cell mass index. In addition, Encover does not significantly affect other dietary conditions based on PG-SGA.
REFERENCES
1. Bistrian BR, Blackburn GL, Hallowell E, Heddle R. 1974; Protein status of general surgical patients. JAMA. 230:858–860. DOI: 10.1001/jama.1974.03240060028025. PMID: 4213823.
2. Kondrup J, Johansen N, Plum LM, Bak L, Larsen IH, Martinsen A, et al. 2002; Incidence of nutritional risk and causes of inadequate nutritional care in hospitals. Clin Nutr. 21:461–468. DOI: 10.1054/clnu.2002.0585. PMID: 12468365.
3. Norman K, Pichard C, Lochs H, Pirlich M. 2008; Prognostic impact of disease-related malnutrition. Clin Nutr. 27:5–15. DOI: 10.1016/j.clnu.2007.10.007. PMID: 18061312.
4. Yu YD, Han JH, Jung SW, Kim DS. 2019; Safety and efficacy of peripheral nutrition fluid (MG-TNA®) in patients undergoing surgery for hepatobiliary and pancreatic disease: results of a phase 4 trial. Ann Hepatobiliary Pancreat Surg. 23:133–137. DOI: 10.14701/ahbps.2019.23.2.133. PMID: 31225414. PMCID: PMC6558125.
5. Nanashima A, Hiyoshi M, Imamura N, Yano K, Hamada T, Hamada R, et al. 2019; Clinical significance of preoperative nutritional parameter and patient outcomes after pancreatectomy: a retrospective study at two academic institute. Ann Hepatobiliary Pancreat Surg. 23:168–173. DOI: 10.14701/ahbps.2019.23.2.168. PMID: 31225419. PMCID: PMC6558137.
6. Correia MI, Waitzberg DL. 2003; The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin Nutr. 22:235–239. DOI: 10.1016/S0261-5614(02)00215-7. PMID: 12765661.
7. Schricker T, Wykes L, Eberhart L, Carli F, Meterissian S. 2005; Randomized clinical trial of the anabolic effect of hypocaloric parenteral nutrition after abdominal surgery. Br J Surg. 92:947–953. DOI: 10.1002/bjs.5105. PMID: 16034820.
8. Shin SH, Kang WH, Han IW, You Y, Lee H, Kim H, et al. 2020; National survey of Korean hepatobiliary-pancreatic surgeons on attitudes about the enhanced recovery after surgery protocol. Ann Hepatobiliary Pancreat Surg. 24:477–483. DOI: 10.14701/ahbps.2020.24.4.477. PMID: 33234751. PMCID: PMC7691206.
9. Fung AKY, Chong CCN, Lai PBS. 2020; ERAS in minimally invasive hepatectomy. Ann Hepatobiliary Pancreat Surg. 24:119–126. DOI: 10.14701/ahbps.2020.24.2.119. PMID: 32457255. PMCID: PMC7271107.
10. Cavallaro P, Bordeianou L. 2019; Implementation of an ERAS pathway in colorectal surgery. Clin Colon Rectal Surg. 32:102–108. DOI: 10.1055/s-0038-1676474. PMID: 30833858. PMCID: PMC6395097.
11. Zaghiyan K, Felder S, Ovsepyan G, Murrell Z, Sokol T, Moore B, et al. 2013; A prospective randomized controlled trial of sugared chewing gum on gastrointestinal recovery after major colorectal surgery in patients managed with early enteral feeding. Dis Colon Rectum. 56:328–335. DOI: 10.1097/DCR.0b013e31827e4971. PMID: 23392147.
12. Tanaka M. 2005; Gastroparesis after a pylorus-preserving pancreatoduodenectomy. Surg Today. 35:345–350. DOI: 10.1007/s00595-004-2961-8. PMID: 15864414.
13. Kang Y, Park S, Kim S, Kim SY, Koh H. 2019; Therapeutic efficacy of exclusive enteral nutrition with specific polymeric diet in pediatric Crohn's disease. Pediatr Gastroenterol Hepatol Nutr. 22:72–79. DOI: 10.5223/pghn.2019.22.1.72. PMID: 30671376. PMCID: PMC6333584.
14. Kong SH, Park JS, Lee IK, Ryu SW, Park YK, Yang HK, et al. 2017; Postoperative oral nutritional supplementation after major gastrointestinal surgery: a randomized controlled clinical trial. Asia Pac J Clin Nutr. 26:811–819. DOI: 10.6133/apjcn.112016.02. PMID: 28802290.
15. Okamura Y, Maeda A, Matsunaga K, Kanemoto H, Uesaka K. 2012; Negative impact of low body mass index on surgical outcomes after hepatectomy for hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 19:449–457. DOI: 10.1007/s00534-011-0461-y. PMID: 21983894.
16. Kim SH, Shon JY, Park JS, Kim JW, Kang JH, Yun EY, et al. 2016; Change in dietary intake and nutritional status using Mealworms as hospital meal in postoperative patients. J Korean Diet Assoc. 22:292–309. DOI: 10.14373/JKDA.2016.22.4.292.
17. Salacinski AJ, Howell SM, Hill DL. 2018; Validity of the InBody 520™ to predict metabolic rate in apparently healthy adults. J Sports Med Phys Fitness. 58:1275–1280. DOI: 10.23736/S0022-4707.17.06719-6. PMID: 28558441.
18. Ogawa H, Fujitani K, Tsujinaka T, Imanishi K, Shirakata H, Kantani A, et al. 2011; InBody 720 as a new method of evaluating visceral obesity. Hepatogastroenterology. 58:42–44. PMID: 21510284.
19. Ntineri A, Prapa S, Bountzona I, Menti A, Stergiou GS. 2021; Validation of the single-cuff oscillometric blood pressure monitor InBody BPBIO750 for public spaces according to the Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization Universal Standard. Blood Press Monit. 26:146–148. DOI: 10.1097/MBP.0000000000000507. PMID: 33323723.
20. De Groot LM, Lee G, Ackerie A, van der Meij BS. 2020; Malnutrition screening and assessment in the cancer care ambulatory setting: mortality predictability and validity of the Patient-Generated Subjective Global Assessment Short form (PG-SGA SF) and the GLIM criteria. Nutrients. 12:2287. DOI: 10.3390/nu12082287. PMID: 32751724. PMCID: PMC7468976.
21. Nitichai N, Angkatavanich J, Somlaw N, Voravud N, Lertbutsayanukul C. 2019; Validation of the Scored Patient-Generated Subjective Global Assessment (PG-SGA) in Thai setting and association with nutritional parameters in cancer patients. Asian Pac J Cancer Prev. 20:1249–1255. DOI: 10.31557/APJCP.2019.20.4.1249. PMID: 31030501. PMCID: PMC6948895.
22. Wiegert EVM, Padilha PC, Peres WAF. 2017; Performance of Patient-Generated Subjective Global Assessment (PG-SGA) in patients with advanced cancer in palliative care. Nutr Clin Pract. 32:675–681. DOI: 10.1177/0884533617725071. PMID: 28850795.
23. Du H, Liu B, Xie Y, Liu J, Wei Y, Hu H, et al. 2017; Comparison of different methods for nutrition assessment in patients with tumors. Oncol Lett. 14:165–170. DOI: 10.3892/ol.2017.6154. PMID: 28693149. PMCID: PMC5494893.
24. Zhang YH, Xie FY, Chen YW, Wang HX, Tian WX, Sun WG, et al. 2018; Evaluating the nutritional status of oncology patients and its association with quality of life. Biomed Environ Sci. 31:637–644. DOI: 10.3967/bes2018.088. PMID: 30369342.
25. You JW, Lee SJ, Kim YE, Cho YJ, Jeong YY, Kim HC, et al. 2013; Association between weight change and clinical outcomes in critically ill patients. J Crit Care. 28:923–927. DOI: 10.1016/j.jcrc.2013.07.055. PMID: 24075294.
26. Ghorabi S, Ardehali H, Amiri Z, Vahdat Shariatpanahi Z. 2016; Association of the adductor pollicis muscle thickness with clinical outcomes in intensive care unit patients. Nutr Clin Pract. 31:523–526. DOI: 10.1177/0884533615621547. PMID: 26869610.
27. Allison SP. 2000; Malnutrition, disease, and outcome. Nutrition. 16:590–593. DOI: 10.1016/S0899-9007(00)00368-3.
Table 1
Clinicopathologic characteristics of subjects
Table 2
Preoperative body cell mass index difference between the experimental group and the control group
Table 3
Body cell mass index at postoperative seven days and outpatient follow-up
Table 4
Change amount in body cell mass index between postoperative seven days and outpatient follow-up




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download