Abstract
When planning pancreaticoduodenectomy for pancreatic head cancer, the prevalence of anatomical variation of the proximal jejunal vein (PJV), the associated short-term surgical outcomes, and the level of PJV convergence to the superior mesenteric vein must be carefully analyzed from both technical and oncological points of view. The prevalence of the first jejunal trunk (FJT) and PJV located ventral to the superior mesenteric artery is 58%–88% and 13%–37%, respectively. Patients with the FJT had a larger amount of intraoperative bleeding and a higher proportion of patients requiring transfusions compared to those without a common trunk. The risk of transfusion was higher in patients with ventral PJV compared to those with dorsal PJV. Although less frequent, sacrificing the FJT can result in fatal venous congestion of the jejunum. Therefore, a well-planned approach for pancreaticoduodenectomy, based on preoperative evaluation of anatomical variation in the PJV, may help reduce intraoperative bleeding and postoperative morbidity. Additionally, the importance of invasion into the PJVs should be revisited in terms of resectability and oncological clearance.
During pancreatic head resection, whether preserved or resected, the major blood vessels around the pancreas are the most important indicators for surgery. Especially, approach to the uncinate process of the pancreas during pancreaticoduodenectomy (PD) requires special attention due to its complex anatomy and proximity to the superior mesenteric artery (SMA), the superior mesenteric vein (SMV), and the proximal jejunal vein (PJV). Dissecting the uncinate process from the SMA and the SMV is one of the steps prone to excessive bleeding that requires meticulous manipulation of the tissue [1]. Various small tributaries to the SMV from the pancreatic head and the uncinate process increase the risk of bleeding. In addition, due to its deep dorsal location, it is difficult to control the bleeding once it occurs. Furthermore, the risk of sacrificing the PJV has been under debate due to the possibility of developing catastrophic congestion of the jejunal limb, which is used for biliary and pancreatic reconstruction during PD [2-4]. Although there are inevitable cases with tumor invasion that require resection of the PJV, much focus is needed to avoid causing unnecessary injury to the PJV. It is important to understand the anatomy of the PJV to reduce both intraoperative bleeding and postoperative morbidities [5,6].
On the other hand, “tumors exceeding the inferior border of the third portion of the duodenum” are classified as “unresectable-locally advanced” tumors in the 7th edition of the Japan Pancreas Society classification [7]. However, little oncological evidence has been presented to define the resectability of tumors that extend below “the inferior border of the third portion of the duodenum”, apart from the technical challenges associated with multiple tributaries of the SMV existing below the inferior border of the third portion of the duodenum.
Therefore, when planning for a PD, the prevalence of anatomical variation of PJV and its associated short-term surgical outcomes, and the level of PJV converging to SMV must be carefully considered from both technical and oncological viewpoints.
The converging pattern of the PJV can be identified in the majority of preoperative imaging studies, preferably, with contrast-enhanced computed tomography accompanied with 3-dimensional vessel reconstruction. Various classifications have been suggested based on the topology and draining territory of the “first jejunal vein” (J1V) [3,8-10], “first jejunal venous trunk” [2,3,11], “proximal dorsal jejunal vein” [12], “left branch of SMV” [13], or “mesenteric branch” [14]. However, no consensus has been reached in nomenclature or classification of the PJV until now.
This review includes a dataset of 136 patients who underwent PD due to pancreatic neoplasm in National Cancer Center, Korea. For consistent description, the anatomical variation of the PJV was reviewed based on the Japanese studies that labeled the order of jejunal veins based on the accompanying jejunal arteries [2]. The J1V was defined as the jejunal vein accompanying the first jejunal artery [3,9]. When the J1V formed a common trunk with the second jejunal vein, the venous trunk was defined as the first jejunal trunk (FJT) [3,9]. The converging pattern of the PJV and the topological relationship between the SMA and the PJV based on the classification, as proposed by Ishikawa et al. [3], was limited to the level of the first-order jejunal veins due to difficulties in identification of J2V. Hence, in our study, type 3-1 and 3-2 in Ishikawa’s classification were classified as type 3, and Ishikawa’s type 3-3 and 3-4 as type 4, i.e., type 1 (FJT located dorsal to the SMA; Fig. 1A), type 2 (FJT located ventral to the SMA; Fig. 1B), type 3 (J1V located dorsal to the SMA; Fig. 1C), and type 4 (J1V located ventral to the SMA; Fig. 1D).
A summary of the prevalence of anatomical variation of PJV in the literature and our dataset is shown in Table 1. According to previous studies, an FJT that drains one or more of the jejunal artery territories was present in 67.0% to 87.8% of all patients, and 63.4% to 87.2% of the PJVs were located dorsal to the SMA [2,3,8-12,15]. Our dataset revealed that 58.1% of the patients had an FJT and 80.1% of the PJVs were located dorsal to SMA.
Recent studies have recognized the technical implications of the PJV during PD [4,5,9,16,17]. The PJV, one of the major tributaries of the SMV, displays complicated anatomical variation in the extent of draining territories and its topology in relation to the SMA (Fig. 2) [3,4,10,16-18]. In particular, the PJV and the inferior pancreaticoduodenal artery (IPDA) are usually embedded in the dorsal side of the mesoduodenum; exposing the PJV to injuries during identification of the IPDA [5]. It is particularly important to fully understand the anatomy of the PJV before approaching the IPDA during “artery-first approach” [5]. Dorsal PJVs can be visualized after dissecting soft tissue between the SMV and the SMA. On the other hand, ventral PJVs conceal the origins of the IPDA, and hence dissecting ventral PJVs from the SMA may cause bleeding from the inferior pancreaticoduodenal veins, of which 79% are drained into the PJV [3]. In the era of laparoscopic PD, it is becoming more important to avoid excessive bleeding around the uncinate process, the SMV, and the SMA than in open surgery.
Among our patients, the volume of intraoperative blood loss (median 450 mL, IQR 290–700 mL [FJT] vs. median 300 mL, IQR 197.5–400 mL [without common trunk], p < 0.01) and proportion of patients requiring a transfusion (12.7%, 10/79 [FJT] vs. 1.8%, 1/57 [without common trunk], p = 0.03) were higher in those patients with FJT compared to those without a common trunk (Table 2). The odds of transfusion were, 8.5 times higher in patients with FJT (95% confidence interval [CI], 1.033–69.460; p < 0.05), and 4.1 times higher in patients with ventral PJVs (95% CI, 1.102–15.386; p = 0.04). According to previous studies, intraoperative blood loss was reduced in J1V oriented mesenteric excision for PD [5]. Therefore, converging patterns of the PJV should be identified preoperatively in order to plan for a safe and effective approach to the area and minimize intraoperative bleeding.
Ischemic insult caused by venous congestion of the jejunal limb after PD has been considered serious due to its remarkable morbidity and mortality [19-22]. Evidence for the actual incidence of jejunal congestion after resection of PJV is limited and is mostly based on case reports. Kobayashi et al. [2] reported that 1 out of 32 patients with PJV resection developed severe congestion of the jejunum requiring urgent jejunal resection. Although it is desirable to preserve PJVs during PD, pancreatic head cancers frequently invade into PJVs. If the first-order jejunal vein needs to be sacrificed, special attention should be paid to not misidentifying the FJT for J1V. The FJT drains the territories of more than two jejunal arteries and is also a main drainage vein of the jejunal limb used for biliopancreatic reconstruction during PD [3]. If mesenteric venous drainage is preserved, segmental resection of one of the two first-order jejunal veins can be considered feasible [4]. Some studies have reported comparable morbidity and mortality rates between patients with and without resection of the jejunal venous trunk or proximal dorsal jejunal vein [2,12]. However, division of the FJT, which drains multiple jejunal veins, can cause severe congestion of the corresponding territories [2]. In addition, a reduced amount of intestinal juice secretion, implying decreased viability of the jejunum, has been reported in patients with a sacrificed FJT, even when there was no significant congestion of the small bowel [2]. It is almost impossible to reconstruct FJT or J1V, unless the caliber is large enough. Elaborate preoperative review of converging patterns of the PJV is essential to minimize the risk of developing extensive venous congestion of the jejunal limb, especially in cases with suspected tumor involvement of the FJT.
Nevertheless, few studies including our dataset identified anatomical variation of the PJV intraoperatively. Although a meta-analysis reported a good correlation in vascular anatomy across radiological, surgical, and cadaveric studies [15], future studies should include better intraoperative identification of PJVs to confirm the findings of preoperative imaging studies.
Despite the definition of “unresectable-locally advanced” tumors in the 7th edition of the Japan Pancreas Society classification [7], 12.5% of the patients who completed PD in our dataset had the PJV converging into the SMV at the level of the inferior border of the third portion of the duodenum. Therefore, it is inappropriate to generalize that all tumors extending below the inferior border of the third portion of the duodenum involve small tributaries of the SMV, going beyond the technical limitations. Consequently, the resectability criteria in relation to the inferior border of the third portion of the duodenum should be revisited.
On the other hand, the proximal dorsal jejunal veins that branch from the dorsal side of the SMV above the inferior border of the duodenum are a frequent site of local invasion because these veins frequently contact the uncinate process of pancreas [12]. Moreover, 81.4% of uncinate process cancer develops local or loco-systemic recurrence around the SMA and SMV [23]. However, a study where these veins were resected routinely in patients undergoing PD for pancreatic cancer reported comparable prognosis in patients with and without tumor invasion into these veins [12]. The result supports the revised National Comprehensive Cancer Network (NCCN) guideline, that omitted the clause “having a contact with most proximal draining jejunal branch into SMV,” which since 2015, had been one of the criteria of unresectable pancreatic head or uncinate process cancer [24,25].
A recent study reported that patients with PJVs that drain ventral to the SMA had poor prognosis and a higher rate of distant metastasis compared to those with PJVs that drain dorsal to the SMA, suggesting that a ventral PJV predisposes tumor spread to SMA in uncinate process cancer [9]. It was hypothesized that the anatomical proximity between ventral PJV and pancreatic head cancer within the uncinate process makes tumors prone to infiltration around the SMA, whereas tumors with dorsal PJV are prone to infiltration around the SMV. Although the current level of evidence is low, the anatomical variation of the PJV from an oncological point of view needs to be understood in more detail.
The prevalence of FJTs and PJVs located ventral to the SMA is 58%–88% and 13%–37%, respectively. Patients with FJT had a higher volume of intraoperative blood loss and proportion of patients requiring transfusion compared to those without a common trunk. Patients with ventral PJV also had an increased risk of transfusion compared to those with dorsal PJV. Although circumstantial, sacrificing FJT results in catastrophic venous congestion of the jejunal limb. Therefore, a well-planned approach for PD, based on preoperative evaluation of anatomical variation in the PJV, may have benefits in reducing intraoperative bleeding and postoperative morbidities. Focus should be on ensuring zero injuries on the FJTs found prior to surgery by mistaking them for J1V or pancreatic veins. Ventral PJV makes the SMA-first approach difficult to perform and care must be taken during dissection of the vessel from the SMA to avoid laceration of the inferior pancreaticoduodenal vein draining into the PJV. In addition, the significance of invasion into the PJV should be re-analyzed in terms of resectability and oncological clearance. Based on both technical and oncological points of view, the SMA-first approach can be a good option for safe dissection and identification of the PJV without increasing the risk of bleeding during PD.
REFERENCES
1. Kawai M, Tani M, Ina S, Hirono S, Nishioka R, Miyazawa M, et al. 2008; CLIP method (preoperative CT image-assessed ligation of inferior pancreaticoduodenal artery) reduces intraoperative bleeding during pancreaticoduodenectomy. World J Surg. 32:82–87. DOI: 10.1007/s00268-007-9305-y. PMID: 18027017.
2. Kobayashi Y, Sakamoto Y, Arita J, Akamatsu N, Kaneko J, Hasegawa K, et al. 2018; Vascular anatomy of the jejunal mesentery and complications associated with division of the first jejunal venous trunk during pancreaticoduodenectomy. J Surg Oncol. 117:1297–1304. DOI: 10.1002/jso.24948. PMID: 29205361.
3. Ishikawa Y, Ban D, Matsumura S, Mitsunori Y, Ochiai T, Kudo A, et al. 2017; Surgical pitfalls of jejunal vein anatomy in pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci. 24:394–400. DOI: 10.1002/jhbp.451. PMID: 28342263.
4. Katz MH, Fleming JB, Pisters PW, Lee JE, Evans DB. 2008; Anatomy of the superior mesenteric vein with special reference to the surgical management of first-order branch involvement at pancreaticoduodenectomy. Ann Surg. 248:1098–1102. DOI: 10.1097/SLA.0b013e31818730f0. PMID: 19092356.
5. Nakamura M, Nakashima H, Tsutsumi K, Matsumoto H, Muta Y, Ueno D, et al. 2013; First jejunal vein oriented mesenteric excision for pancreatoduodenectomy. J Gastroenterol. 48:989–995. DOI: 10.1007/s00535-012-0697-6. PMID: 23076543.
6. Shukla PJ, Barreto SG, Kulkarni A, Nagarajan G, Fingerhut A. 2010; Vascular anomalies encountered during pancreatoduodenectomy: do they influence outcomes? Ann Surg Oncol. 17:186–193. DOI: 10.1245/s10434-009-0757-1. PMID: 19838756.
7. Japan Pancreas Society. Classification of pancreatic carcinoma. 4th English ed. Tokyo: Kanehara & Co.;2017. DOI: 10.1245/s10434-009-0757-1.
8. Hamabe A, Park S, Morita S, Tanida T, Tomimaru Y, Imamura H, et al. 2018; Analysis of the vascular interrelationships among the first jejunal vein, the superior mesenteric artery, and the middle colic artery. Ann Surg Oncol. 25:1661–1667. DOI: 10.1245/s10434-018-6456-z. PMID: 29616421.
9. Nishimura S, Takahashi H, Akita H, Asukai K, Hasegawa S, Yamada D, et al. 2019; The anatomical pattern of the proximal jejunal vein as a prognostic factor in patients with pancreatic head cancer treated with preoperative chemoradiation therapy. Anticancer Res. 39:5821–5830. DOI: 10.21873/anticanres.13786. PMID: 31570487.
10. Sakaguchi T, Suzuki S, Morita Y, Oishi K, Suzuki A, Fukumoto K, et al. 2010; Analysis of anatomic variants of mesenteric veins by 3-dimensional portography using multidetector-row computed tomography. Am J Surg. 200:15–22. DOI: 10.1016/j.amjsurg.2009.05.017. PMID: 20074695.
11. Kim HJ, Ko YT, Lim JW, Lee DH. 2007; Radiologic anatomy of the superior mesenteric vein and branching patterns of the first jejunal trunk: evaluation using multi-detector row CT venography. Surg Radiol Anat. 29:67–75. DOI: 10.1007/s00276-006-0153-5. PMID: 17033735.
12. Hosokawa Y, Nagakawa Y, Sahara Y, Takishita C, Nakajima T, Hijikata Y, et al. 2018; Surgical outcomes of pancreaticoduodenectomy for pancreatic cancer with proximal dorsal jejunal vein involvement. J Gastrointest Surg. 22:1179–1185. DOI: 10.1007/s11605-018-3722-0. PMID: 29520646.
13. Graf O, Boland GW, Kaufman JA, Warshaw AL, Fernandez del Castillo C, Mueller PR. 1997; Anatomic variants of mesenteric veins: depiction with helical CT venography. AJR Am J Roentgenol. 168:1209–1213. DOI: 10.2214/ajr.168.5.9129413. PMID: 9129413.
14. Horton KM, Fishman EK. 2002; Volume-rendered 3D CT of the mesenteric vasculature: normal anatomy, anatomic variants, and pathologic conditions. Radiographics. 22:161–172. DOI: 10.1148/radiographics.22.1.g02ja30161. PMID: 11796905.
15. Negoi I, Beuran M, Hostiuc S, Negoi RI, Inoue Y. 2018; Surgical anatomy of the superior mesenteric vessels related to pancreaticoduodenectomy: a systematic review and meta-analysis. J Gastrointest Surg. 22:802–817. DOI: 10.1007/s11605-018-3669-1. PMID: 29363018.
16. Inoue Y, Saiura A, Yoshioka R, Ono Y, Takahashi M, Arita J, et al. 2015; Pancreatoduodenectomy with systematic mesopancreas dissection using a supracolic anterior artery-first approach. Ann Surg. 262:1092–1101. DOI: 10.1097/SLA.0000000000001065. PMID: 25587814.
17. Takemura N, Miki K, Kosuge T. 2016; New portal-superior mesenteric vein reconstructions using first jejunal vein flap in pancreaticoduodenectomy. World J Surg. 40:1462–1466. DOI: 10.1007/s00268-016-3426-0. PMID: 26801505.
18. Papavasiliou P, Arrangoiz R, Zhu F, Chun YS, Edwards K, Hoffman JP. 2012; The anatomic course of the first jejunal branch of the superior mesenteric vein in relation to the superior mesenteric artery. Int J Surg Oncol. 2012:538769. DOI: 10.1155/2012/538769. PMID: 22489264. PMCID: PMC3303700.
19. Huťan M, Bartko C, Slyško R, Sekáč J, Prochotský A, Majeský I, et al. 2014; Superior mesenteric vein thrombosis - unusual management of unusual complication of Whipple procedure. Int J Surg Case Rep. 5:765–768. DOI: 10.1016/j.ijscr.2014.09.004. PMID: 25255475. PMCID: PMC4189080.
20. Katz MH, Lee JE, Pisters PW, Skoracki R, Tamm E, Fleming JB. 2012; Retroperitoneal dissection in patients with borderline resectable pancreatic cancer: operative principles and techniques. J Am Coll Surg. 215:e11–e18. DOI: 10.1016/j.jamcollsurg.2012.05.015. PMID: 22818108. PMCID: PMC4637976.
21. Kayashima H, Maeda T, Harada N, Masuda T, Ohmine T, Yamaguchi S, et al. 2016; One-step surgery for acute ischemia of the jejunal loop after pancreatoduodenectomy: report of a case. Surg Case Rep. 2:24. DOI: 10.1186/s40792-016-0153-6. PMID: 26976614. PMCID: PMC4791446.
22. Zyromski NJ, Howard TJ. 2008; Acute superior mesenteric-portal vein thrombosis after pancreaticoduodenectomy: treatment by operative thrombectomy. Surgery. 143:566–567. DOI: 10.1016/j.surg.2007.10.020. PMID: 18374055.
23. Kim JR, Kim H, Kwon W, Jang JY, Kim SW. 2021; Pattern of local recurrence after curative resection in pancreatic ductal adenocarcinoma according to the initial location of the tumor. J Hepatobiliary Pancreat Sci. 28:105–114. DOI: 10.1002/jhbp.854. PMID: 33084211.
24. National Comprehensive Cancer Network. 2020. Pancreatic adenocarcinoma (version 1.2020) [Internet]. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1455. cited 2020 Mar 19. Plymouth Meeting: National Comprehensive Cancer Network.
25. National Comprehensive Cancer Network. 2015. Pancreatic adenocarcinoma (version 2.2015) [Internet]. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1455. cited 2015 Dec 1. Plymouth Meeting: National Comprehensive Cancer Network.
Fig. 1
Computed tomography images of the proximal jejunal vein anatomy. (A) First jejunal trunk (FJT) located dorsal to the superior mesenteric artery (SMA) (type 1). (B) FJT located ventral to the SMA (type 2). (C) First jejunal vein (J1V) located dorsal to the SMA (type 3). (D) J1V located ventral to the SMA (type 4). The arrows indicate proximal jejunal veins with each type of anatomical variation.
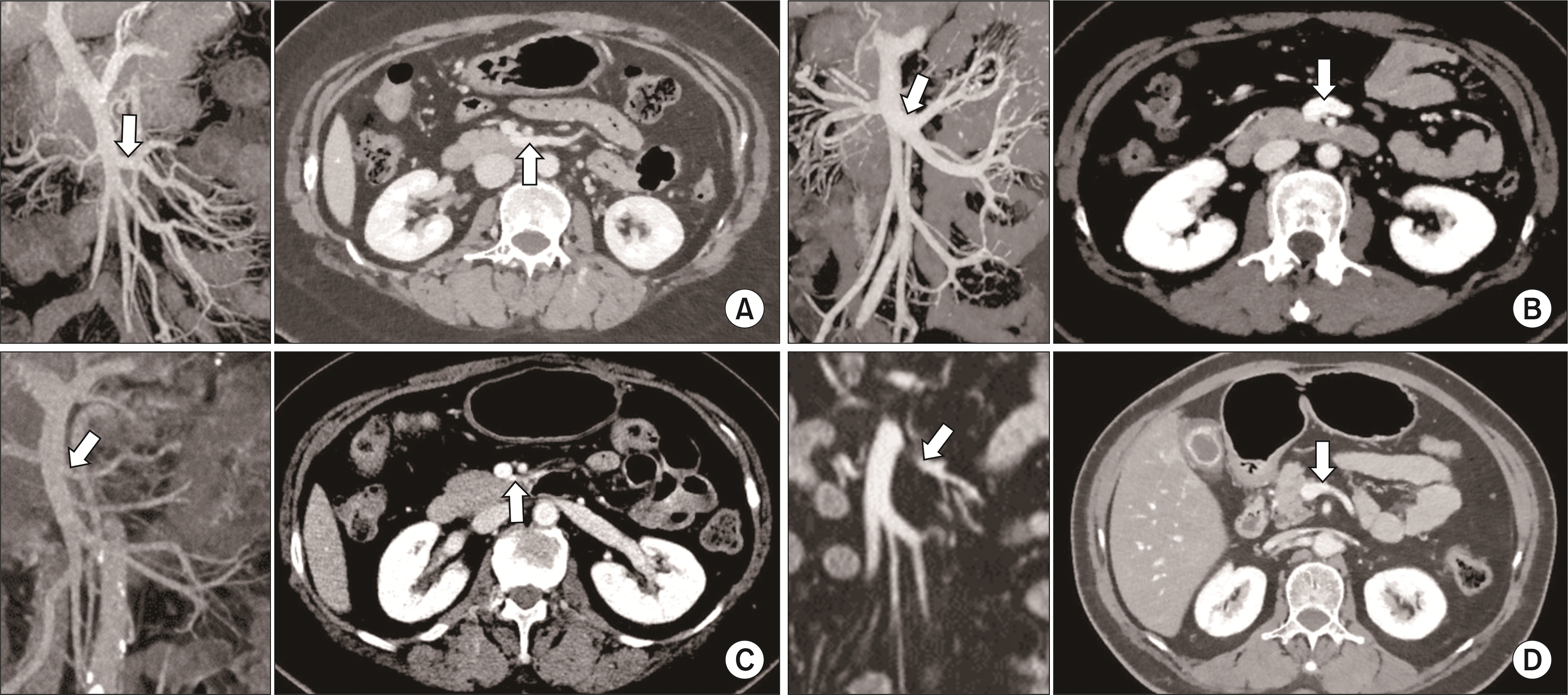
Fig. 2
An illustration of the relationship between proximal jejunal vein (PJV) and pancreatic head cancer. (A) PJV located dorsal to the SMA. Dorsal PJV can get injured during identification of the inferior pancreaticoduodenal artery, which is embedded in the dorsal side of the mesoduodenum. (B) PJV located ventral to the SMA. Dissecting ventral PJV from the SMA may cause bleeding from the inferior pancreaticoduodenal veins, which are drained into the PJV. SMA, superior mesenteric artery; SMV, superior mesenteric vein.
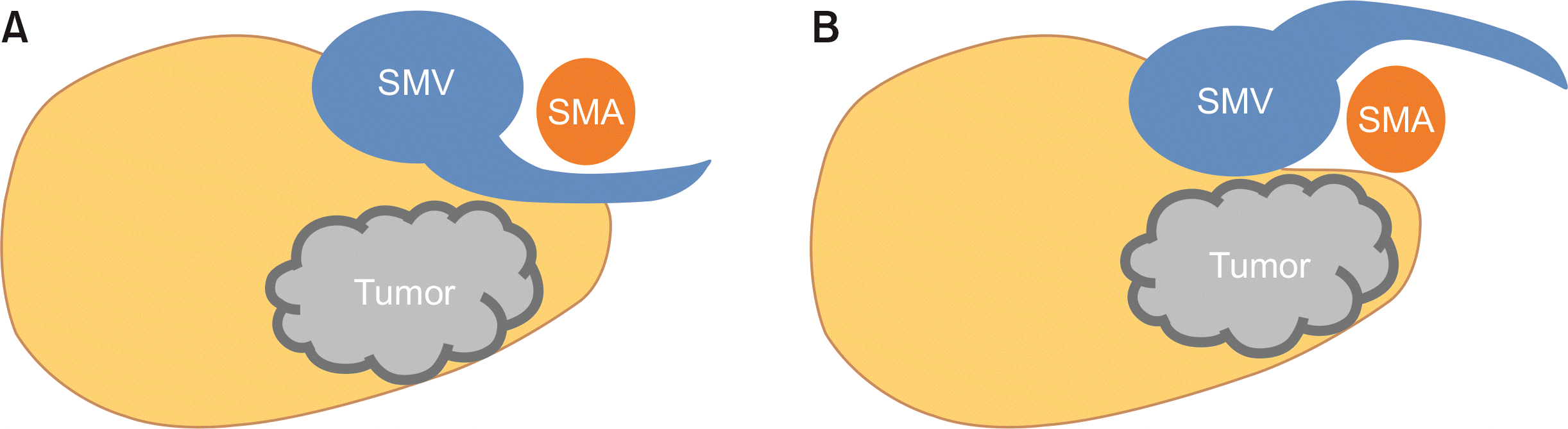
Table 1
The prevalence of anatomical variation of proximal jejunal vein (PJV)
| Anatomical variation | Ishikawa et al. [3] | Hosokawa et al. [12] | Kobayashi et al. [2] | Present study |
|---|---|---|---|---|
| No. of patients | 155 | 121 | 123 | 136 |
| Type 1 (dorsal FJT) | 98 (63.2) | 74 (61.2) | 72 (58.5) | 63 (46.3) |
| Type 2 (ventral FJT) | 32 (20.6) | 7 (5.8) | 36 (29.3) | 16 (11.8) |
| Type 3-1 (dorsal J1V – dorsal J2V) | 7 (4.5) | 21 (17.3) | 6 (4.9) | 46 (33.8) |
| Type 3-2 (dorsal J1V – ventral J2V) | 9 (5.8) | 3 (2.5) | ||
| Type 3-3 (ventral J1V – dorsal J2V) | 5 (3.2) | 16 (13.2) | 9 (7.3) | 11 (8.1) |
| Type 3-4 (ventral J1V – ventral J2V) | 4 (2.6) | 0 (0) | ||
| FJT (%) | 83.9 | 67.0 | 87.8 | 58.1 |
| Dorsal PJV (%) | 73.5 | 81.0 | 63.4 | 80.1 |
Table 2
Perioperative characteristics based on the anatomical types of proximal jejunal vein
| Variable | Dorsal FJT (n = 63) | Ventral FJT (n = 16) | Dorsal J1V (n = 46) | Ventral J1V (n = 11) | p-valuea) |
|---|---|---|---|---|---|
| Operation | 0.13 | ||||
| PD | 21 (33.3) | 9 (56.3) | 15 (32.6) | 4 (36.4) | |
| PPPD | 36 (57.1) | 6 (37.5) | 31 (67.4) | 6 (54.5) | |
| PrPD | 6 (9.5) | 1 (6.3) | 0 (0) | 1 (9.1) | |
| Portal vein resection | 15 (23.8) | 3 (18.8) | 8 (17.4) | 3 (27.3) | 0.80 |
| Operation time (min) | 340 (300–390) | 355 (303–390) | 335 (305–370) | 345 (305–380) | 0.80 |
| Estimated blood loss (mL) | 450 (270–700) | 425 (295–700) | 300 (195–400) | 400 (200–600) | < 0.01 |
| Transfusion | 6 (9.5) | 4 (25.0) | 0 (0) | 1 (9.1) | < 0.01 |
| Transfusion unit | 4 (2–4) | 4 (2–6) | 0 | 2 | 0.71 |
| Postoperative complication | 32 (50.8) | 10 (62.5) | 19 (41.3) | 5 (45.5) | 0.50 |
| ≥Grade III complication | 12 (19.0) | 3 (18.8) | 4 (8.7) | 3 (27.3) | 0.27 |
| Postoperative pancreatic fistula | 10 (15.9) | 2 (12.5) | 8 (17.4) | 3 (27.3) | 0.78 |
| Biochemical leak | 2 (3.2) | 1 (6.3) | 5 (10.9) | 0 (0) | |
| Grade B | 7 (11.1) | 1 (6.3) | 3 (6.5) | 3 (27.3) | |
| Grade C | 1 (1.6) | 0 (0) | 0 (0) | 0 (0) | |
| Pseudoaneurysm bleeding | 3 (4.8) | 0 (0) | 1 (2.2) | 2 (18.2) | 0.15 |
| Delayed gastric emptying | 8 (12.7) | 3 (18.8) | 0 (0) | 1 (9.1) | 0.02 |
| Portal vein stent insertion | 2 (3.2) | 0 (0) | 0 (0) | 0 (0) | 0.68 |
| Postoperative hospital stay (day) | 21 (16–28) | 17 (14–21) | 16 (14–21) | 18 (14–27) | 0.12 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download