This article has been
cited by other articles in ScienceCentral.
Abstract
Backgrounds/Aims
Historically, incidence and survival analysis and annual traits for primary liver cancer (LC) has not been investigated in a population-based study in Korea. The purpose of the current study is to determine incidence, survival rate of patients with primary LC in Korea.
Methods
We conducted a retrospective cohort study using Korea Central Cancer Registry based on the Korea National Cancer Incidence Database. Statistical analysis including crude rate and age-standadized rate (ASR) of incidence and mortality was performed for LC patients registered with C22 code in International Classification of Diseases, tenth revision from 1999 to 2019. Subgroup analysis was performed for hepatocellular carcinoma (HCC, C22.0) and intrahepatic cholangiocarcinoma (IHCC, C22.1).
Results
The crude incidence rate of HCC (21.0 to 22.8 per 100,000) and IHCC (2.3 to 5.6 per 100,000) increased in the observed period from 1999 to 2019. The ASR decreased in HCC (20.7 to 11.9 per 100,000) but remained unchanged in IHCC (2.4 to 2.7 per 100,000). The proportion of HCC patients diagnosed in early stages (localized or regional Surveillance, Epidemiology, and End Results or SEER stage) increased significantly over time. As expected, 5-yeat survival rate of HCC was greatly improved, reaching 42.4% in the period between 2013 and 2019. This trait was more prominent in localized SEER stage. On the other hand, the proportion of IHCC patients diagnosed in localized stage remained unchanged (22.9% between 2013 and 2019), although ASR and 5-year survival rate showed minor improvements.
Conclusions
A great improvement in survival rate was observed in patients with newly diagnosed HCCs. It was estimated to be due to an increase in early detection rate. On the contrary, detection rate of an early IHCC was stagnant with a minor improvement in prognosis.
Keywords: Liver cancer, Hepatocellular carcinoma, Intrahepatic cholangiocarcinoma, SEER stage
INTRODUCTION
Liver cancer (LC) is the sixth most commonly diagnosed cancer and the fourth leading cause of cancer-related deaths worldwide. In 2018, it was estimated that approximately 841,080 individuals were newly diagnosed with LC, which was responsible for 781,631 deaths [
1]. The highest incidence rates of LC were observed in countries in Eastern Asia including Korea [
2]. LC has two distinct histologic classifications: hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (IHCC). HCC comprised approximately 70% to 85% of all LC cases and IHCC comprised the remaining 10% to 20% cases [
3]. Recent advances in effective prophylaxis, surveillance, and treatment of viral hepatitis and resultant liver cirrhosis have played a role in decreasing the incidence of HCC [
4]. Moreover, introduction of novel diagnostic and surgical techniques has made a major impact on improvement of survival of HCC patients [
5]. However, this is far from the case of IHCC in Korea. The incidence of IHCC is still increasing with a stagnant early detection rate [
6]. To further improve survival and reduce socioeconomic cost resulting from LC, understanding the current status of nationwide LC incidence and survival rates as well as changes in traits over the past decades is of a great importance. Thus, the aim of this study was to stratify the incidence and survival rates of LC using the most trustworthy national database. Results of this study will provide clinicians with baseline information for envisioning future advances in LC treatment.
MATERIALS AND METHODS
Data source
Epidemiologic data of primary LCs were obtained from the Korea Central Cancer Registry (KCCR) based on the Korea National Cancer Incidence Database [
7]. The KCCR database is one of the most reliable sources of population based cancer registry database in Korea. It currently covers over 90% of new nationwide cancer cases [
8]. Patients registered as C22 (LC) according to the International Classification of Diseases, tenth revision (ICD-10) from 1999 to 2019 were included in this study [
9]. Subgroup analysis was performed for HCC (C22.0), IHCC (C22.1), and other LCs (C22.2–C22.9). The total study period of 1999 to 2019 was divided into three distinct periods for convenience of interpretation on cancer incidence and survival rate traits: period I, 1999–2005; period II, 2005–2011; and period III, 2011–2019.
The Surveillance, Epidemiology, and End Results (SEER) stage has been published by KCCR since 2006. SEER stage at diagnosis was classified as localized (invasive cancer limited to the organ of origin), regional (tumor extension beyond the limits of organ of origin), distant disease (spread to distant areas from the primary tumor), and unknown [
10]. According to SEER staging guideline, localized LCs are defined as LCs confined to liver, either single tumor without vascular invasion or multiple tumors confined to one lobe without vascular invasion. LCs with regional extension are defined as those with invasion to surrounding organs or tissues including diaphragm, extrahepatic bile duct(s), extrahepatic blood vessels (i.e., hepatic artery, portal vein and vena cava), gallbladder, the lesser omentum, or peritoneum. LCs involving both lobes of the liver or single lobe with vascular invasion or regional lymph nodes are also included in regional diseases. Regional lymph nodes are defined as those at the liver hilum. Any LCs extending beyond the above-mentioned structures or baring distant metastasis are defined as distant diseases. Information on SEER stage of LC has been available in KCCR database since 2006.
The first course of treatment was grouped into three categories, referring to documented cancer-directed treatment methods within the first four months of the date of initial diagnosis [
10]: surgical (surgery alone, surgery with chemotherapy, surgery with radiotherapy, surgery with chemotherapy and radiotherapy), non-surgical (chemotherapy alone, chemotherapy with radiotherapy, radiotherapy alone), and no active or unknown treatment information [
11]. Transcatheter arterial chemoembolization (TACE) for HCC was included in the non-surgical treatment group. The informed consent was waived in this retrospective study.
Statistical analysis
The study period was divided into periods I (1999–2005), II (2006–2012), and III (2013–2019). Rates were expressed as crude rate (CR) and age-standardized rate (ASR) per 100,000 individuals. CR was calculated as the total number of incidence/mortality cases divided by the mid-year population of the specified year. ASR was the weighted average of ASR standardized using Segi’s world standard population [
12]. Annual percent change (APC) of ASRs and average APC (AAPC) as a weighted average of APCs for the whole study period were computed. Relative survival rates were estimated using the Ederer II method with some minor modifications [
13] based on an algorithm written in SAS provided by Paul Dickman in Karolinska Institutet, Stockholm, Sweden. All statistical tests were two-tailed. Results were considered statistically significant when p-value was less than 0.05. All statistical analyses were performed using SAS 9.4 (SAS Institute, Inc., Cary, NC, USA) and Joinpoint 4.7.0.0 (National Cancer Institute, Bethesda, MD, USA).
RESULTS
Baseline characteristics of patients with liver cancers
From 1999 to 2019, a total of 319,963 individuals were newly diagnosed with primary LC, which comprised 8.3% (n = 3,844,605) of all cancers in Korea. Among them, 242,605 (75.8%) were HCCs and 50,422 (15.8%) were IHCCs. Annual cases of primary LC increased from 1999 to 2011. They tended to decrease thereafter until 2019. In 1999, a total of 13,262 cases of LC were found. The annual case number peaked in 2011 with 16,418 cases newly diagnosed. In 2019, however, the number of new LC cases decreased to 15,605. The CR of incidence of LC during the observed period from 1999 to 2019 showed an increasing trend (28.1 in 1999 to 30.4 per 100,000 in 2019;
Fig. 1A). However, ASRs of both incidence and mortality of LC were reduced in great numbers. In 2019, ASRs of incidence and mortality of LC were 15.6 and 9.9, respectively (
Supplementary Table 1, 2).
Fig. 1
Overall annual cases age-standardized crude rates and male-to-female ratio of LC. (A) Overall annual cases of LC. (B) Age-standardized crude rates of LC. (C) Male-to-female ASR of LC. LC, liver cancer; HCC, hepatocellular carcinoma; IHCC, intrahepatic cholangiocarcinoma; ASR, age-standardized rate.
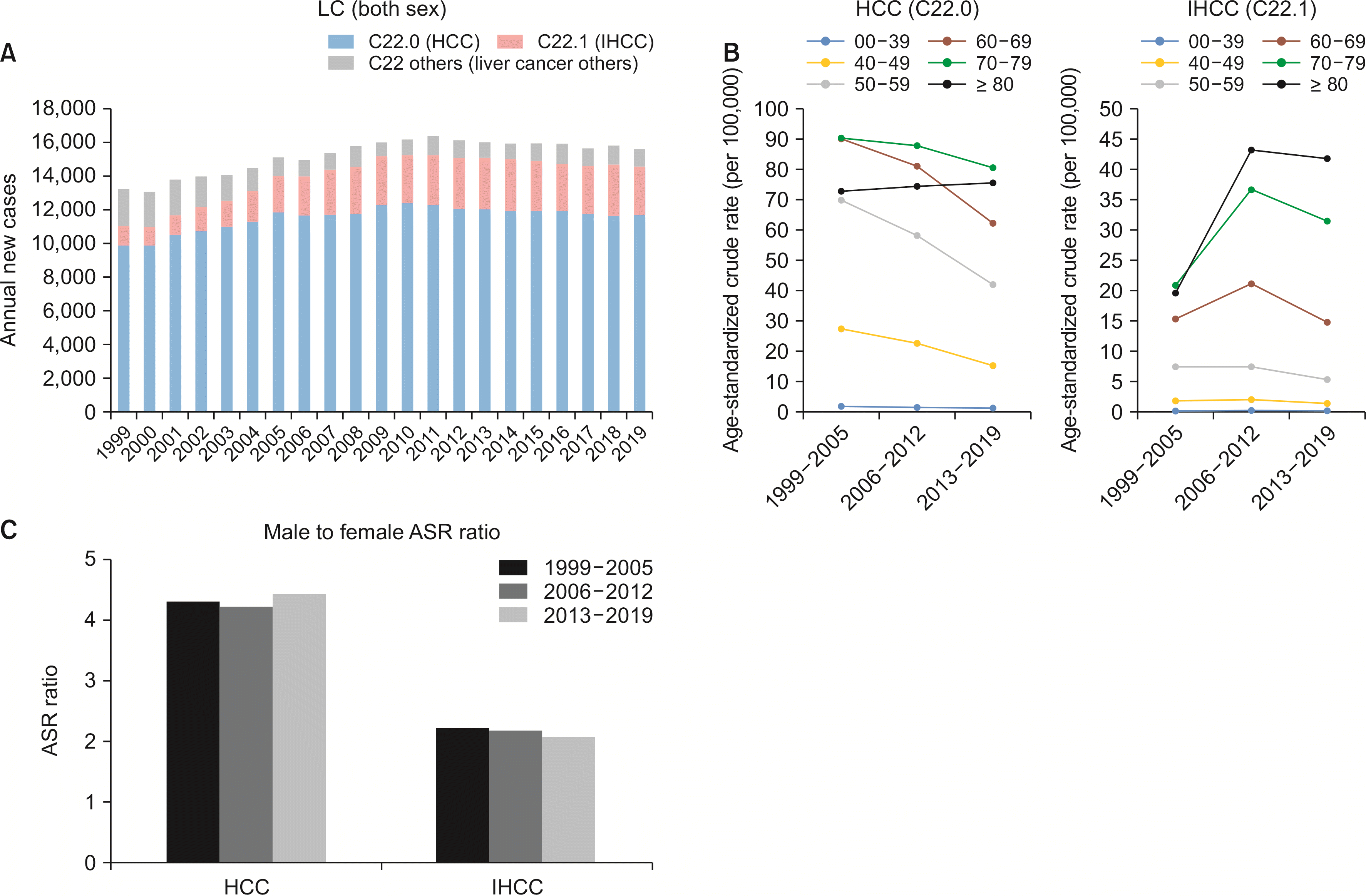

The incidence of HCC peaked in age group from 50 to 59 years, although 51.2% of HCC patients were diagnosed at the age of 60 years or older. In comparison, the incidence of IHCC tended to increase with age, with 75.3% of newly diagnosed IHCC patients being older than 60 years of age (
Table 1,
Fig. 1B). Both malignancies were diagnosed predominantly in male gender (In HCCs and IHCCs, male patients accounted for 78.3% and 61.8%, respectively). As expected, male to female ASR ratio in HCC was as high as 4.32 in period I, 4.23 in period II, and 4.43 in period III, which remained stable throughout the total study period. Male predominance of ASR ratio in IHCC was less prominent. It was 2.23 in period I, 2.20 in period II, and 2.08 in period III (
Fig. 1C).
Table 1
Demographics of liver cancer in Korea
|
Demographic |
HCC (C22.0) (n = 242,605) |
IHCC (C22.1) (n = 50,422) |
C22 others (liver cancer others) (n = 26,936) |
C22 (liver) (n = 319,963) |
|
Age (yr) |
|
|
|
|
|
00–39 |
8,051 (3.3) |
712 (1.4) |
1,130 (4.2) |
9,893 (3.1) |
|
40–49 |
37,579 (15.5) |
2,970 (5.9) |
2,715 (10.1) |
43,264 (13.5) |
|
50–59 |
72,610 (29.9) |
8,761 (17.4) |
5,844 (21.7) |
87,215 (27.3) |
|
60–69 |
66,291 (27.3) |
14,819 (29.4) |
7,233 (26.9) |
88,343 (27.6) |
|
70–79 |
43,221 (17.8) |
15,636 (31.0) |
6,673 (24.8) |
65,530 (20.5) |
|
≥ 80 |
14,853 (6.1) |
7,524 (14.9) |
3,341 (12.4) |
25,718 (8.0) |
|
Sex |
|
|
|
|
|
Male |
190,067 (78.3) |
31,182 (61.8) |
18,256 (67.8) |
239,505 (74.9) |
|
Female |
52,538 (21.7) |
19,240 (38.2) |
8,680 (32.2) |
80,458 (25.1) |
|
First course of treatment |
|
|
|
|
|
Any surgery |
40,027 (16.5) |
11,565 (22.9) |
4,750 (17.6) |
56,342 (17.6) |
|
Non-surgical |
99,474 (41.0) |
10,607 (21.0) |
5,174 (19.2) |
115,255 (36.0) |
|
No active treatment/ Unknown |
103,104 (42.5) |
28,250 (56.0) |
17,012 (63.2) |
148,366 (46.4) |
|
Unknown |
|
|
|
|
|
SEER Stage (2006–2019) |
|
|
|
|
|
Localized |
83,322 (49.8) |
9,398 (23.4) |
4,608 (31.7) |
97,328 (43.8) |
|
Regional |
37,488 (22.4) |
11,222 (28.0) |
2,259 (15.6) |
50,969 (23.0) |
|
Distant |
17,888 (10.7) |
12,612 (31.5) |
2,409 (16.6) |
32,909 (14.8) |
|
Unstaged |
28,732 (17.2) |
6,875 (17.1) |
5,243 (36.1) |
40,850 (18.4) |

Surgery-first approach within 4 months after initial diagnosis was performed in 16.5% of HCC patients and 22.9% of IHCC patients. Alternative treatments including systemic chemotherapy, TACE, and radiotherapy were performed in 41.0% of HCC patients and 21.0% of IHCC patients. Approximately half of HCC patients (49.8%) were diagnosed at localized SEER stage, whereas only 23.4% of IHCC patients were diagnosed at localized stage (
Table 1).
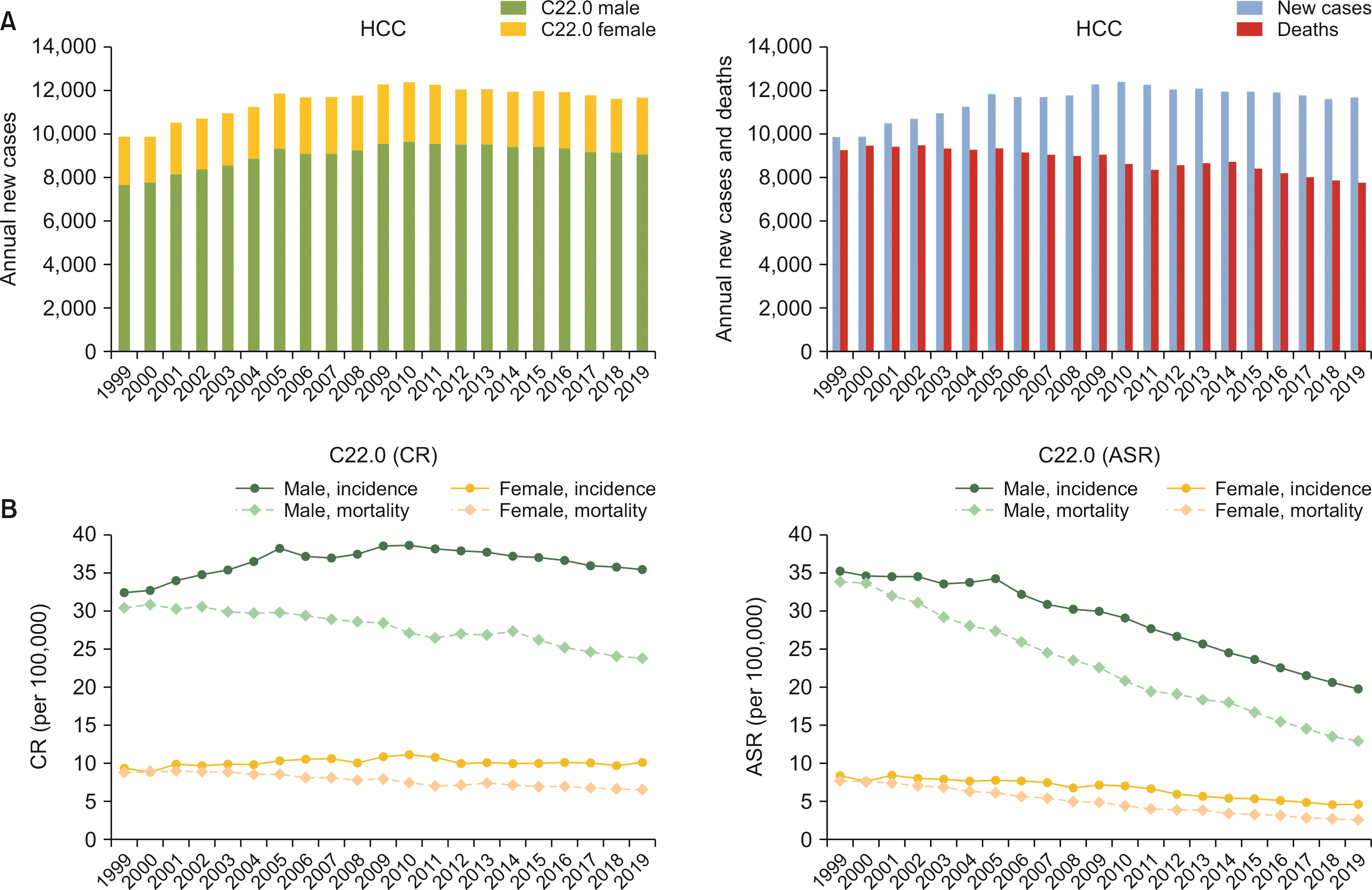
Hepatocellular carcinoma
The annual number of new HCC cases increased from 9,894 to 12,293 from 1999 to 2011, but decreased to 11,698 in 2019. Cancer deaths from HCC continuously decreased from 9,290 in 1999 to 7,794 in 2019 (
Fig. 2A,
Supplementary Table 3, 4). HCC was diagnosed predominantly in males, with a constant male to female ASR ratio of 4.2 from 2006 to 2021 and 4.4 from 2013 to 2019. The CR of incidence in male gender followed the annual incidence rate as it peaked in 2011 by 38.2 per 10,000 and decreased to 35.5 per 10,000 in 2019. However, in females, the trait was less prominent. It remained relatively constant (9.5 per 10,000 in 1999; 10.8 per 10,000 in 2011; and 10.1 per 10,000 in 2019). The ASR of HCC incidence was significantly decreased in both sexes. The AAPC in males was steadily decreased from –0.79 (95% confidence interval [CI]: –1.15, –0.44;
p = 0.02) in period I to –4.22 (95% CI: –4.43, –4.02;
p < 0.01) in period III. From 1999 to 2019, AAPC in males was –2.83 (95% CI: –3.00, –2.66;
p < 0.01). Likewise, AAPC in females was as low as –3.10 (95% CI: –3.61, –2.60;
p < 0.01) during the whole study period from 1999 to 2019. Decreases in mortality rate in both sexes were also notable during the study period. The ASR of mortality from HCC decreased from 19.5 in 1999 to 7.4 in 2019 (males from 34.0 in 1999 to 12.9 in 2019, females from 7.7 in 1999 to 2.6 in 2019). The AAPC of mortality rate from HCC was –3.59 (95% CI: –4.20, –2.99;
p < 0.01) in period I, –5.44 (95% CI: –6.24, –4.63;
p < 0.01) in period II, and –6.40 (95% CI: –7.25, –5.54;
p < 0.01) in period III (
Fig. 2B).
Fig. 2
Annual cases, CR, and ASR of incidence and mortality of HCC. (A) Annual cases and deaths of HCC. (B) CR and ASR of incidence and mortality of HCC. HCC, hepatocellular carcinoma; CR, crude rate; ASR, age-standardized rate.

Between 2006 and 2019, 49.8% of HCC patients had localized diseases and 22.4% had regional diseases (
Table 1). Proportions of both localized and regional diseases increased from period I to period II. However, the percentage of patients with unknown stage decreased (
Fig. 3A). The percentage of surgical treatment being performed as the first-line treatment increased from 16.9% in period I to 22.1% in period II. This trait was extremely prominent in localized SEER stage as it went up from 24.7% to 33.4%. In patients with regional disease of HCC, the proportion of surgical treatment performed slightly increased from 12.3% in period I to 14.6% in period II. However, the surgical treatment rate remained constant in the distant disease SEER stage group (
Fig. 3B).
Fig. 3
Stage, treatments, and survival of hepatocellular carcinoma (HCC). (A) SEER (Surveillance, Epidemiology, and End Results) stage of HCC. (B) First course of treatment for HCC. (C) Five-year survival rate of HCC by year, age, and SEER stage. (D) Five-year survival rate of HCC by SEER stage and treatments. Statistically significant (*p < 0.05; **p < 0.01).
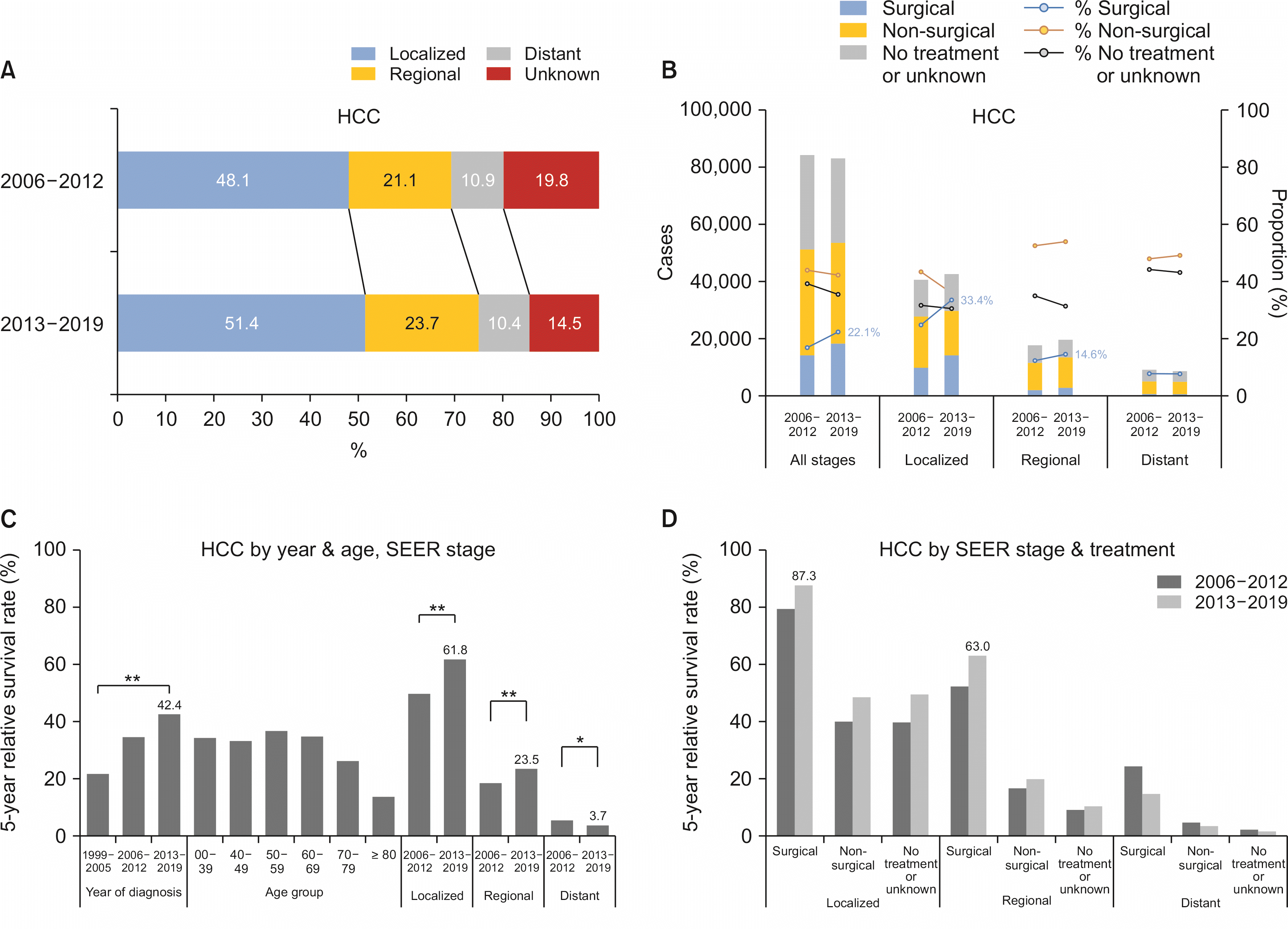

The median survival was 33 months in all stages throughout the study period. Patients diagnosed in localized, regional, and distant SEER stages showed median survival rates of 85, 12, and 3 months, respectively. Meanwhile, the 5-year survival rate greatly increased over time. It increased from 21.7% in period I to 42.4% in period III (
p < 0.01). The increase in survival rate was observed in both localized (
p < 0.01) and regional (
p < 0.01) SEER stage groups from period II to III (localized: 79.2% to 87.3%,
p < 0.01; regional: 52.3% to 63.0%,
p < 0.01). This trait of increase in 5-year survival rate was identically observed in surgery-first and non-surgical treatment groups (
Fig. 3C). From period II to period III, the 5-year survival rate of localized SEER stage patients treated with surgery-first strategy increased from 79.2% to 87.3% (
p < 0.01) and that of regional SEER stage patients treated with surgery significantly increased from 52.3% to 63.0% (
p < 0.01) (
Fig. 3D).
Intrahepatic cholangiocarcinoma
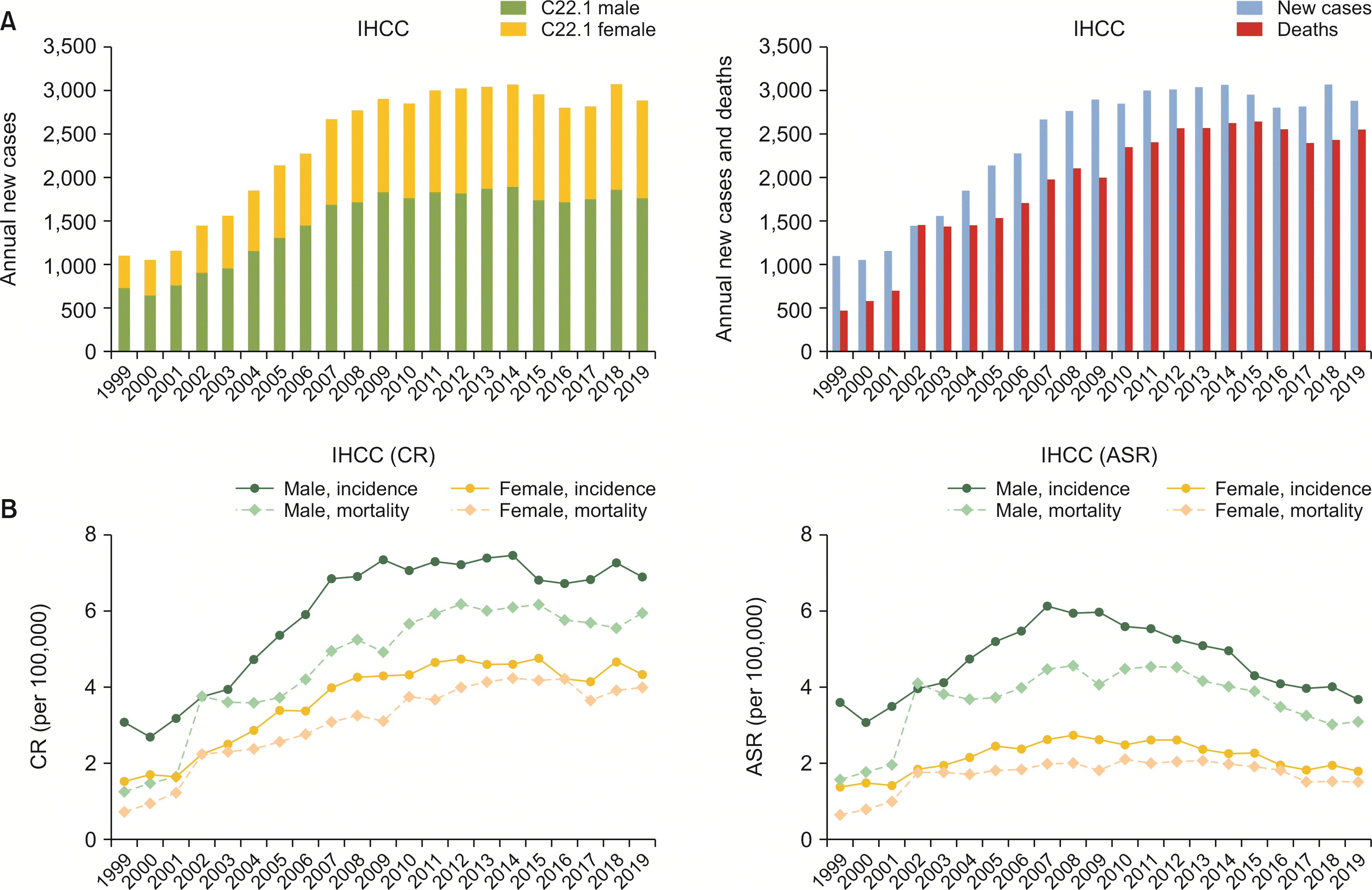
The annual number of new IHCC cases in Korea steadily increased from 1999 to 2009. It plateaued thereafter until 2019 in both sexes. In 1999, 1,102 new cases of IHCC were diagnosed. Annual incidence increased to 2,899 in 2009 and remained stable until 2014 (3,066 new cases of IHCC). The incidence decreased in a small number after 2014, reaching 2,883 cases in 2019. Mortality related to IHCC also followed an identical trend. In 1999, there were 474 deaths related to IHCC. In 2019, 2,551 deaths were witnessed (
Fig. 4A,
Supplementary Table 5, 6). IHCC was diagnosed predominantly in males, with male to female ASR ratio of 2.08 in period III. The CR of incidence in male gender for IHCC was 3.1 per 100,000 in 1999. It increased to 6.9 per 100,000 in 2019. However, the ASR of male gender was decreased to 3.7 per 100,000 after peaking in 2007, which was comparable to that in 1999 (3.6 per 100,000). The CR of mortality from IHCC in male gender was also increased, whereas the ASR of mortality in males decreased to 3.1 per 100,000 in 2019 after peaking in 2010 (4.5 per 100,000). This trait was similar in females. The CR of incidence in females increased from 1.6 per 100,000 in 1999 to 4.3 per 100,000 in 2019, with an AAPC of 4.82 (95% CI: 2.77, 6.91;
p < 0.01), while the ASR of incidence in females remained stable. ASR of mortality in females in 2019 was 1.5 per 100,000 (
Fig. 4B).
Fig. 4
Annual cases, CR, and ASR of incidence and mortality of IHCC. (A) Annual cases by sex, new cases, and deaths of IHCC. (B) CR and aASR of incidence and mortality of IHCC. IHCC, intrahepatic cholangiocarcinoma; CR, crude rate; ASR, age-standardized rate.

Between 2013 and 2019 (period III), 50.3% of newly diagnosed IHCC patients had localized and regional SEER stages and 34.0% had distant SEER stage. The proportion of distant SEER stage slightly increased from 28.7% in period II, while unknown SEER stage decreased in proportion from 18.7% in period II to 15.7% in period III. The percentage of IHCC patients undergoing surgical treatment remained at 22.2% in period III (22.5% in period II). On the other hand, in subgroup analysis for localized SEER stage, the proportion of patients who underwent surgical treatment increased from 33.0% to 41.0% from period II to period III (
Fig. 5A).
Fig. 5
Stage, treatments, and survival of intrahepatic cholangiocarcinoma (IHCC). (A) SEER (Surveillance, Epidemiology, and End Results) stage of IHCC. (B) First course of treatment of IHCC. (C) Five-year survival rate of IHCC by year, age, and SEER stage. (D) Five-year survival rate of IHCC by SEER stage and treatments. Statistically significant (*p < 0.05; **p < 0.01).
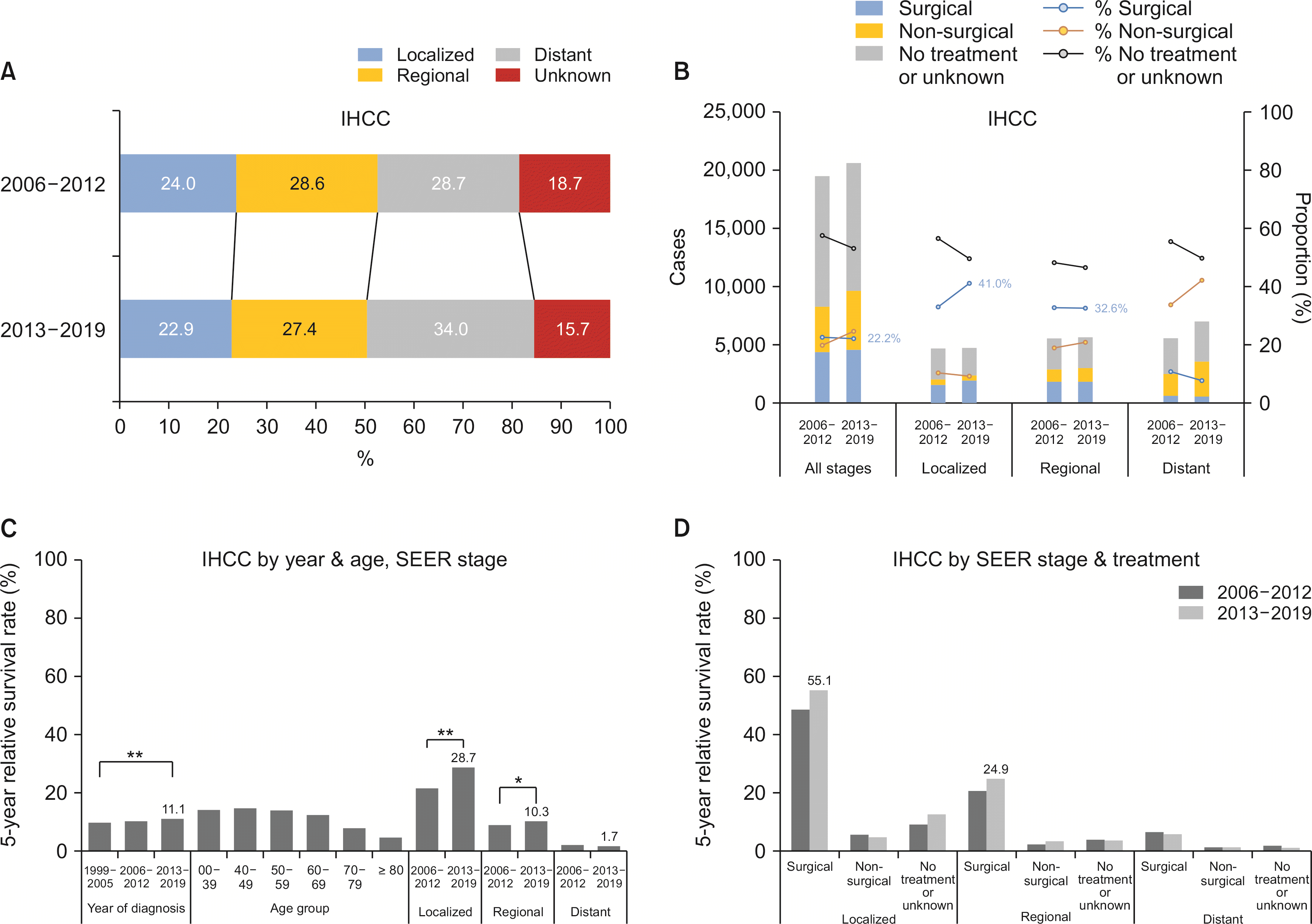

Five-year survival rate after diagnosis of IHCC increased from 9.8% in period I to 10.2% in period II and 11.1% in period III. This change was statistically significant (
p < 0.01). The greatest increase in 5-year survival rate was noted in the localized SEER stage. It increased from 21.5% in period II to 28.7% in period III (
p < 0.01). Likewise, a small increase in 5-year survival rate in regional SEER stage was seen (9.0% in period II to 10.3% in period III,
p < 0.05). In subgroup analysis according to initial treatments, a major increase in 5-year survival rate was noted in the surgical group for localized and regional SEER stage IHCC patients (
Fig. 5B).
DISCUSSION
Nationwide statistics on incidence and survival of LC from 1999 to 2019 is described in this study. In Korea, ASRs of incidence and mortality of LC were significantly decreased during the observed period from 1999 to 2019. In 2019, the ASR of incidence of LC was 15.6 per 100,000 and the ASR of mortality from LC was 9.9 per 100,000, which was comparable to that of developed regions of the world. ASRs of incidence and mortality worldwide were 15.3 and 14.3 per 100,000 in 2012, respectively [
14]. The CR of LC, on the other hand, was stagnant or unchanged. The CR of incidence of LC increased from 28.1 per 100,000 in 1999 to 30.4 per 100,000 in 2019. The discrepancy between CR and ASR was assumed to be due to an increase in life expectancy over time. The life expectancy of the general population has grown rapidly around the globe in the past decades. This trend was extremely prominent in Korea [
15]. Although ASR is used to compare trends of cancer incidence over time, CR is more helpful in estimating cancer burden for a given population [
16].
HCC showed a marked improvement in prognosis during the observed period. ASRs of both incidence and mortality were greatly reduced in both sexes. An increase in early detection rate, and resultant increase in number of patients undergoing radical resection might have been the major factor contributing to this change. Our data showed relevant data as individuals diagnosed of HCC in localized SEER stage increased from 48.1% in period II to 51.4% in period III. Also, the rate of patients undergoing surgery as first course of treatment increased from 16.9% in period II to 22.1% in period III. This was marked in localized SEER stage, as the rate of surgical treatment increased from 24.7% in period II to 33.4% in period III. As a result, 5-year survival rates of HCC in all stages were significantly improved, reaching 42.4% in period III. The 5-year survival rate of localized SEER stage alone was 61.8% in period III.
On the other hand, IHCC in Korea showed a rapid increase in the number in terms of CR and ASR from 1999 to 2007. This initial was considered to be due to topographic misclassification of hilar cholangiocarcinoma or Klatskin tumor (M8162/3). In this article, the statistics followed institutions’ original registration either as a malignancy originating form intrahepatic bile duct (22.1) or an extrahepatic bile duct. Overestimation of intrahepatic bile duct cancer incidence might be responsible for the initial increase in IHCC incidence as described in a previous study [
17]. Unlike cancer registry data with morphology codes, such information is unavailable in mortality statistics. Hence, it is impossible to reclassify “Klatskin tumor (M8162/3)” to either intra- or extra-hepatic bile duct cancer in mortality statistics. Therefore, it should be noticed that the incidence and mortality statistics presented in this review might have been overestimated than real data. However, the ICD-O-3.2 which has been in effect for cancer registration in Korea since 2021 classifies “Klatskin tumor (M8162/3)” only as extrahepatic bile duct cancer. Consequently, cancer registration data will provide more accurate data of extrahepatic bile duct cancer without additional adjustment.
It was also noteworthy that the early detection rate of IHCC did not increase over time. The proportion of localized SEER stage decreased from 24.0% in period II to 22.9% in period III. This result, however, must be interpreted with caution since the proportion of unknown stage also decreased from 18.7% to 15.7%. Nevertheless, our data revealed an unchanged surgical treatment rate in all stages. Data also showed a great improvement in 5-year survival rate in individuals with IHCC who underwent surgery as the first course of treatment. From this dataset, an effective nationwide screening program for IHCC can be recommended. For example, a yearly abdominal ultrasound in persons aged 50 years of age or older covered by national health insurance system can be employed. Current guidelines of Korean National Cancer Screening program grant benefit to individuals aged 40 years or older who are HBsAg positive to receive abdominal ultrasound and serum alpha-feto-protein test in regular checkup.
This study has several limitations. Firstly, thorough longitudinal treatment information was unavailable in the cancer registry data. The effect of surgery as first course of treatment might have been overestimated as alternative non-surgical treatments such as chemotherapy, chemoembolization, and radiotherapy might have been performed after cancer recurrence. The role and effectiveness of each treatment and surgery remain unclear. Also, those treatment modalities in non-surgical entity (chemoembolization in particular) can be performed as bridge treatment before liver transplantation. Such patients could have been classified as ones receiving non-surgical treatment. Thus, survival data can be biased. Secondly, the survival data might have failed to recognize individuals with LC who died from liver failure as underlying liver cirrhosis progressed. Moreover, LC patients who were unable to receive aggressive anti-cancer treatment due to compromised liver function might have been included in the unknown or no treatment group. As such, the survival of surgical treatment group might have been overestimated.
In conclusion, the incidence of HCC decreased in terms of CR and ASR. A great improvement in survival rate was observed in patients with newly diagnosed HCCs. This might be due to an increase in early detection rate. On the contrary, detection rate of an early or localized SEER stage IHCC was stagnant, although ASR and 5-year survival rate showed minor improvements. Further studies are needed to find effective measures for detecting early stage IHCC patients who might benefit from surgical treatments.





