Introduction
Materials and Methods
Chemicals and antibodies
Cell culture and osteogenic differentiation
Alizarin red S staining
Detection of intracellular ROS
RNA isolation, cDNA synthesis, and real time RT-PCR analysis
Western blotting
Statistical analysis
Results
Effects of 3, 4’-DHF on eADSCs during osteogenic differentiation
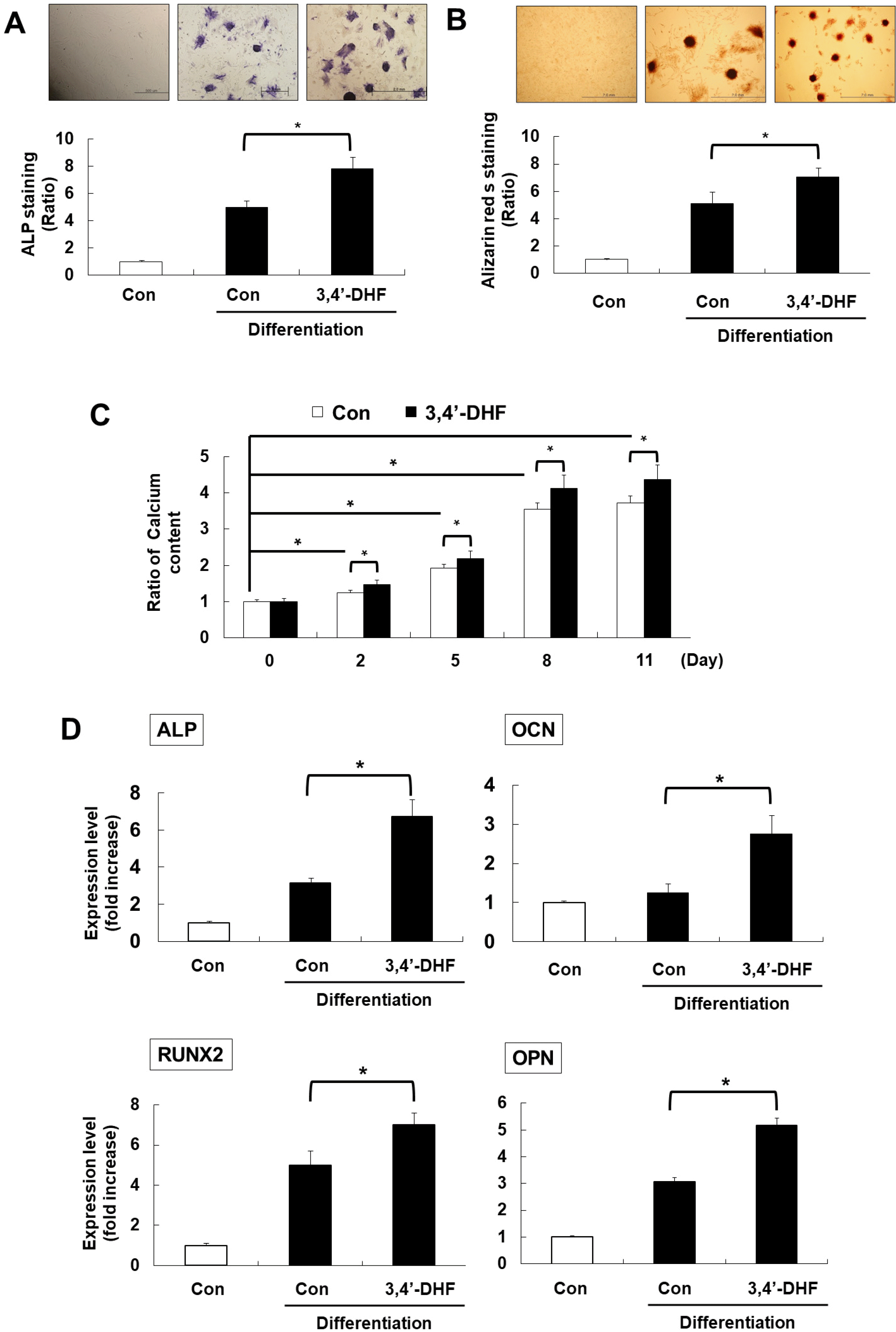 | Fig. 13, 4-’dihydroxyflavone (3, 4’-DHF) enhanced osteogenesis in equine Adipose-Derived Stromal Cells (eADSCs). (A, B) Osteogenic differentiation marker staining of 3, 4’-DHF-treated eADSCs with osteogenic differentiation at day 14 (A) Alkaline phosphatase (ALP) and (B) Alizarin red s. (C) Calcium content ration of 3, 4’-DHF-treated eADSCs with osteogenic differentiation. (D) qRT-PCR analysis of osteogenesis markers (Osteocalcin (OCN), Osteopontin (OPN), RUNX2, and ALP) in eADSCs and 3, 4’-DHF eADSCs. Error bars represent±SD from at least three independent experiments (*p<0.05). |
ERK phosphorylation increased with 3, 4’-DHF treatment during osteogenic differentiation
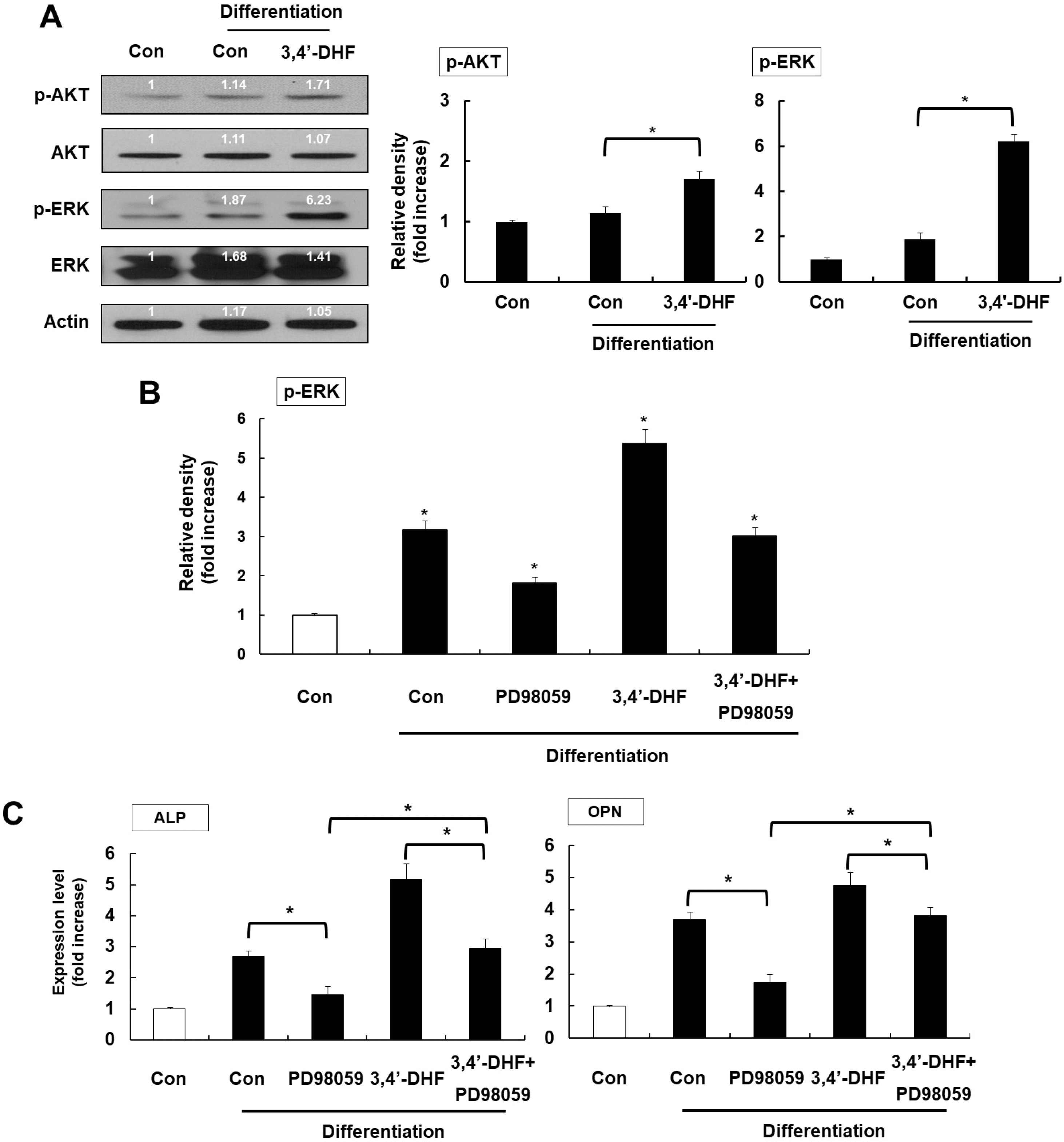 | Fig. 23, 4’-DHF induced ERK activation during osteogenic differentiation. (A) Western blot analysis of ERK and AKT phosphorylation during osteogenic differentiation in the presence or absence of 3, 4’-DHF. (B) Expression level of phosphorylated ERK in the presence or absence of PD98059 or 3, 4’-DHF. (C) qRT-PCR analysis of osteogenic differentiation markers ALP and OPN in eADSCs with or without 3, 4’-DHF-treatment in the presence or absence of PD98059. Each experiment was repeated in triplicate and data are presented as means±standard deviation (p<0.05, denoted by*). |
U0126 induces osteogenic differentiation via BMP signaling despite EKR inactivation in eADSCs
 | Fig. 3Treatment with the ERK inhibitor U0126, led to an increase in osteogenesis in eADSCs and 3, 4’-DHF eADSCs via BMP signaling. (A) Western blot analysis of phosphorylated ERK in eADSCs and 3, 4’-DHF eADSCs in the presence or absence of U0126. (B) qRT-PCR of osteogenesis marker gene expression in eADSCs and 3, 4’-DHF eADSCs treated with U0126. (C) qRT-PCR analysis of BMP2 and BMP4 gene expression in the presence or absence of U0126 and PD98059 in eADSCs and 3, 4’-DHF eADSCs. Error bars represent±SD from at least three independent experiments (*p<0.05).
|
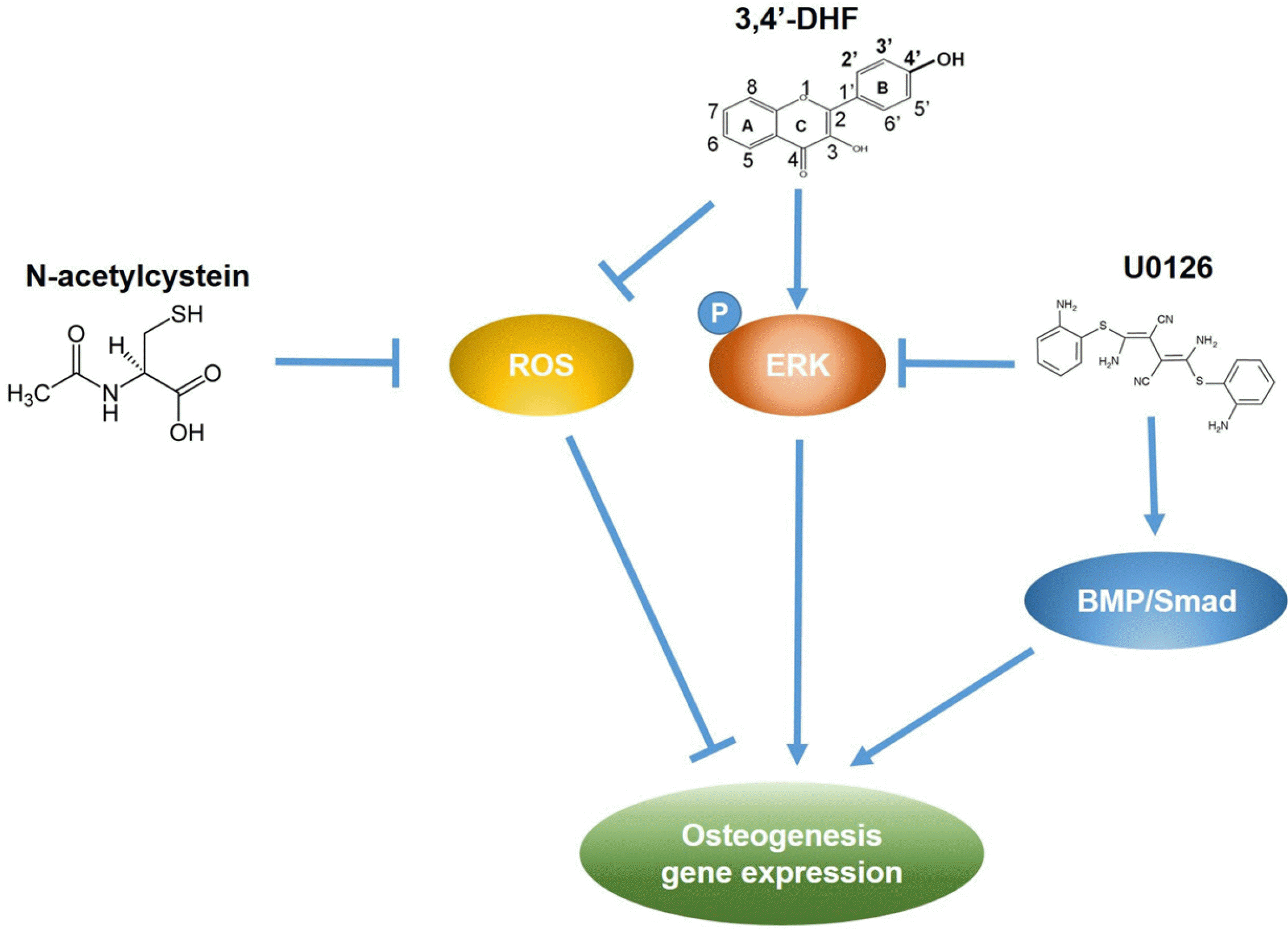
3, 4’-DHF regulates osteogenic differentiation by modulation of ROS signaling
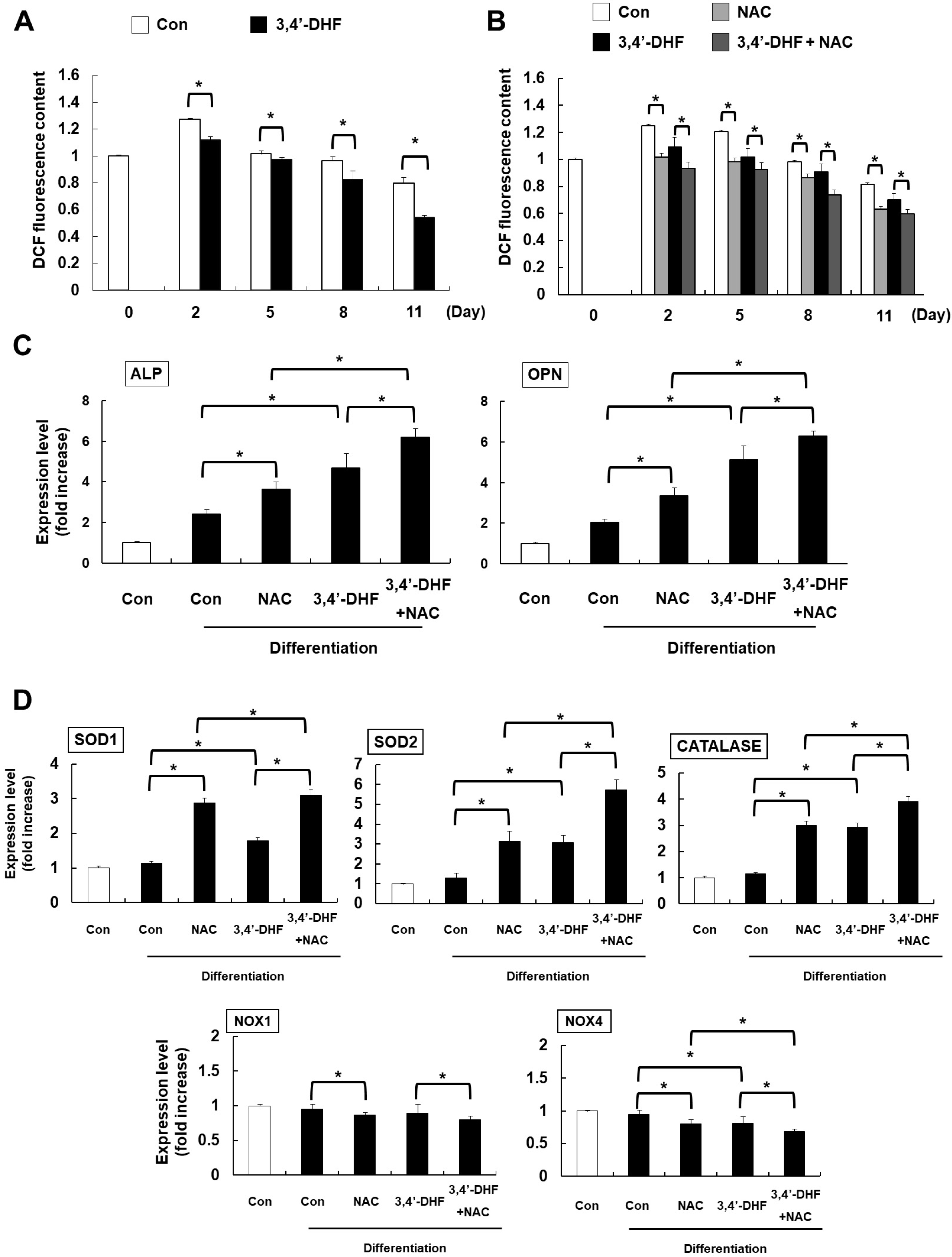 | Fig. 43, 4’-DHF regulates osteogenic differentiation by modulation of Reactive Oxygen Species (ROS) signaling. (A) Intracellular ROS level according to 2’, 7’-dichlorodihydrofluorescein diacetate (H2DCFDA) fluorescence by flow cytometry. (B) The intensity of H2DCFDA fluorescence in N-acetyl cysteine (NAC) treated eADSCs and 3, 4’-DHF eADSCs. (C) Expression of osteogenesis marker genes in NAC treated or untreated eADSCs and 3, 4’-DHF eADSCs according to qRT-PCR analysis. (D) Expression of ROS-related genes in eADSCs and 3, 4ʹ-DHF eADSCs in the presence or absence of NAC treatment. Error bars represent±SD from the mean of three independent experiments (*p<0.05). |
Co-treatments 3, 4’-DHF, U0126 and NAC enhances osteogenic differentiation in eADSCs
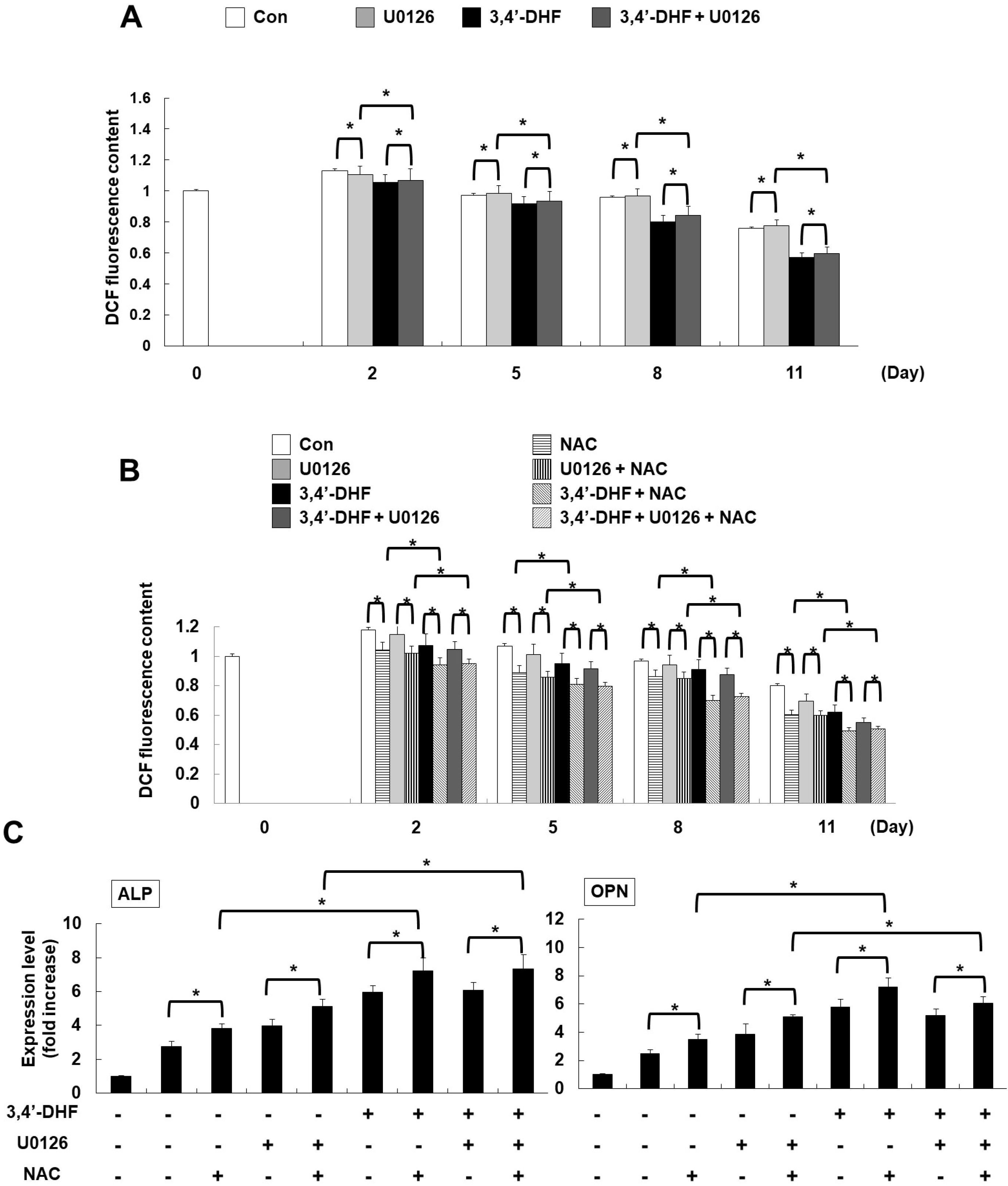 | Fig. 5Co-treatment with 3, 4’-DHF, U0126, and NAC regulated osteogenic differentiation. (A) ROS levels were determined by measuring H2DCFDA fluorescence using a flow cytometer, with or without U0126 treatment in eADSCs or 3, 4’-DHF-treated eADSCs during osteogenic differentiation. (B) Intracellular ROS levels were measured following treatment with U0126 or NAC in eADSCs and 3, 4’-DHF eADSCs. (C) qRT-PCR analysis of osteogenic differentiation marker gene expressions in eADSCs and 3, 4’-DHF eADSCs, in the presence or absence with U0126 or NAC. Each experiment was repeated in triplicate and data are presented as means±SD (*p<0.05). |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download