1. Bantz SK, Zhu Z, Zheng T. 2014; The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. J Clin Cell Immunol. 5:202. DOI:
10.4172/2155-9899.1000202. PMID:
25419479. PMCID:
PMC4240310.
4. Kakinuma T, Nakamura K, Wakugawa M, Mitsui H, Tada Y, Saeki H, Torii H, Asahina A, Onai N, Matsushima K, Tamaki K. 2001; Thymus and activation-regulated chemokine in atopic dermatitis: serum thymus and activation-regulated chemokine level is closely related with disease activity. J Allergy Clin Immunol. 107:535–541. DOI:
10.1067/mai.2001.113237. PMID:
11240957. PMID:
https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0035098042&origin=inward.
8. Shigemoto-Kuroda T, Oh JY, Kim DK, Jeong HJ, Park SY, Lee HJ, Park JW, Kim TW, An SY, Prockop DJ, Lee RH. 2017; MSC-derived extracellular vesicles attenuate immune responses in two autoimmune murine models: type 1 diabetes and uveoretinitis. Stem Cell Reports. 8:1214–1225. DOI:
10.1016/j.stemcr.2017.04.008. PMID:
28494937. PMCID:
PMC5425726. PMID:
https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85019447215&origin=inward.
24. Xia Y, You XE, Chen H, Yan YJ, He YC, Ding SZ. 2017; Epidermal growth factor promotes mesenchymal stem cell-mediated wound healing and hair follicle regeneration. Int J Clin Exp Pathol. 10:7390–7400. PMID:
31966581. PMCID:
PMC6965296.
36. Choi JH, Kim HG, Jin SW, Han EH, Khanal T, Do MT, Hwang YP, Choi JM, Chun SS, Chung YC, Jeong TC, Jeong HG. 2013; Topical application of Pleurotus eryngii extracts inhibits 2,4-dinitrochlorobenzene-induced atopic dermatitis in NC/Nga mice by the regulation of Th1/Th2 balance. Food Chem Toxicol. 53:38–45. DOI:
10.1016/j.fct.2012.11.025. PMID:
23200891. PMID:
https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84871464276&origin=inward.
38. Jeong NH, Yang EJ, Jin M, Lee JY, Choi YA, Park PH, Lee SR, Kim SU, Shin TY, Kwon TK, Jang YH, Song KS, Kim SH. 2018; Esculetin from Fraxinus rhynchophylla attenuates atopic skin inflammation by inhibiting the expression of inflammatory cytokines. Int Immunopharmacol. 59:209–216. DOI:
10.1016/j.intimp.2018.04.005. PMID:
29656211. PMID:
https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85045418520&origin=inward.
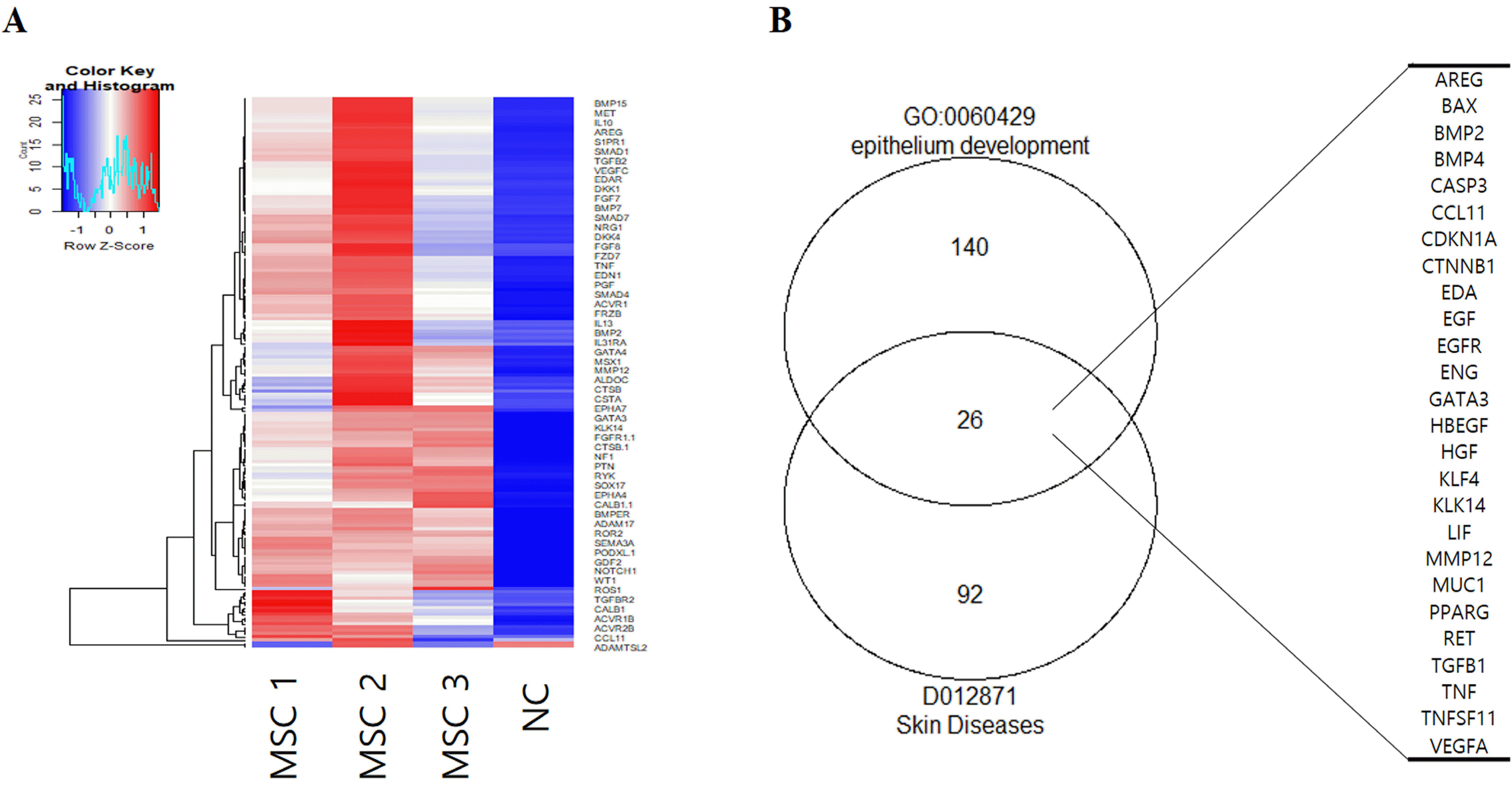
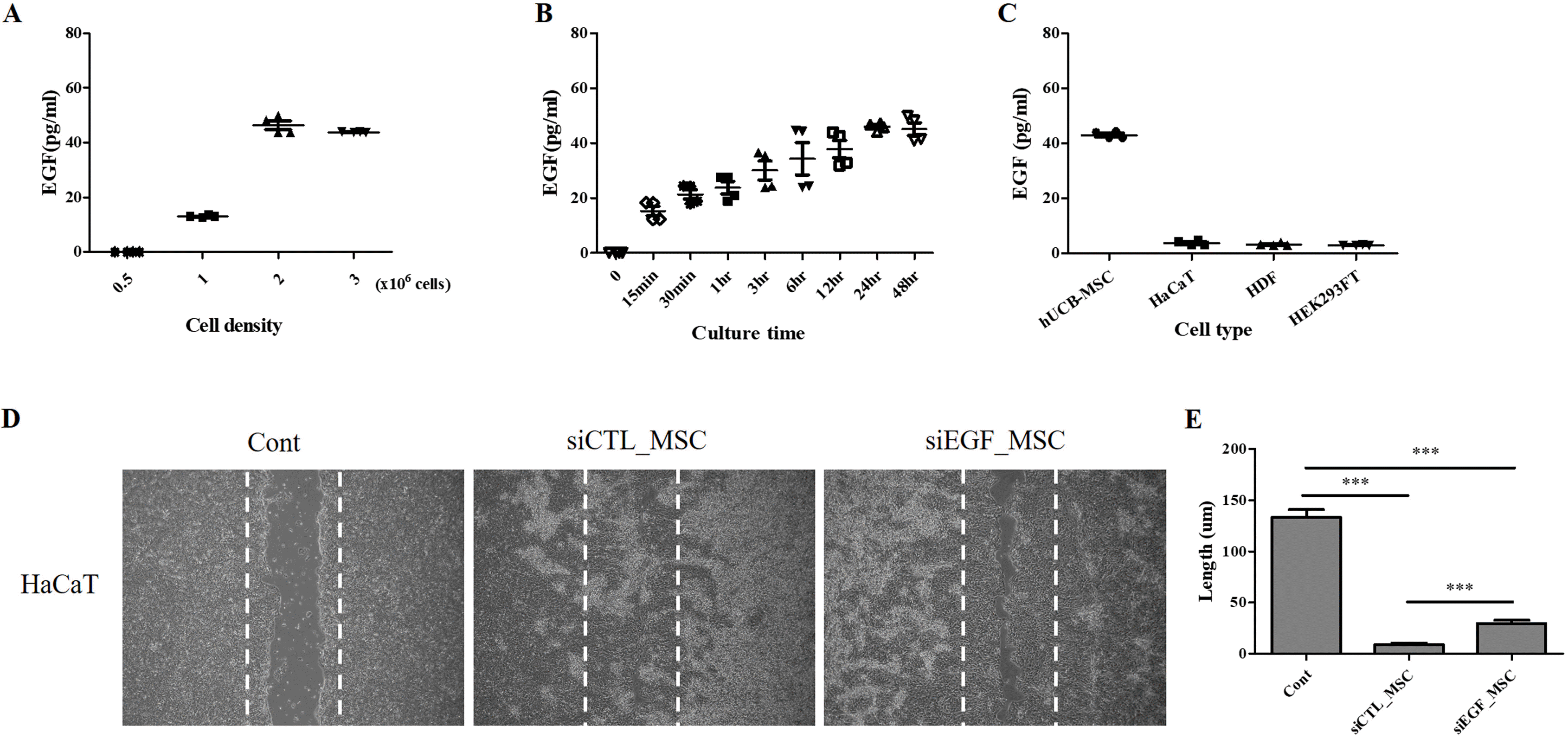
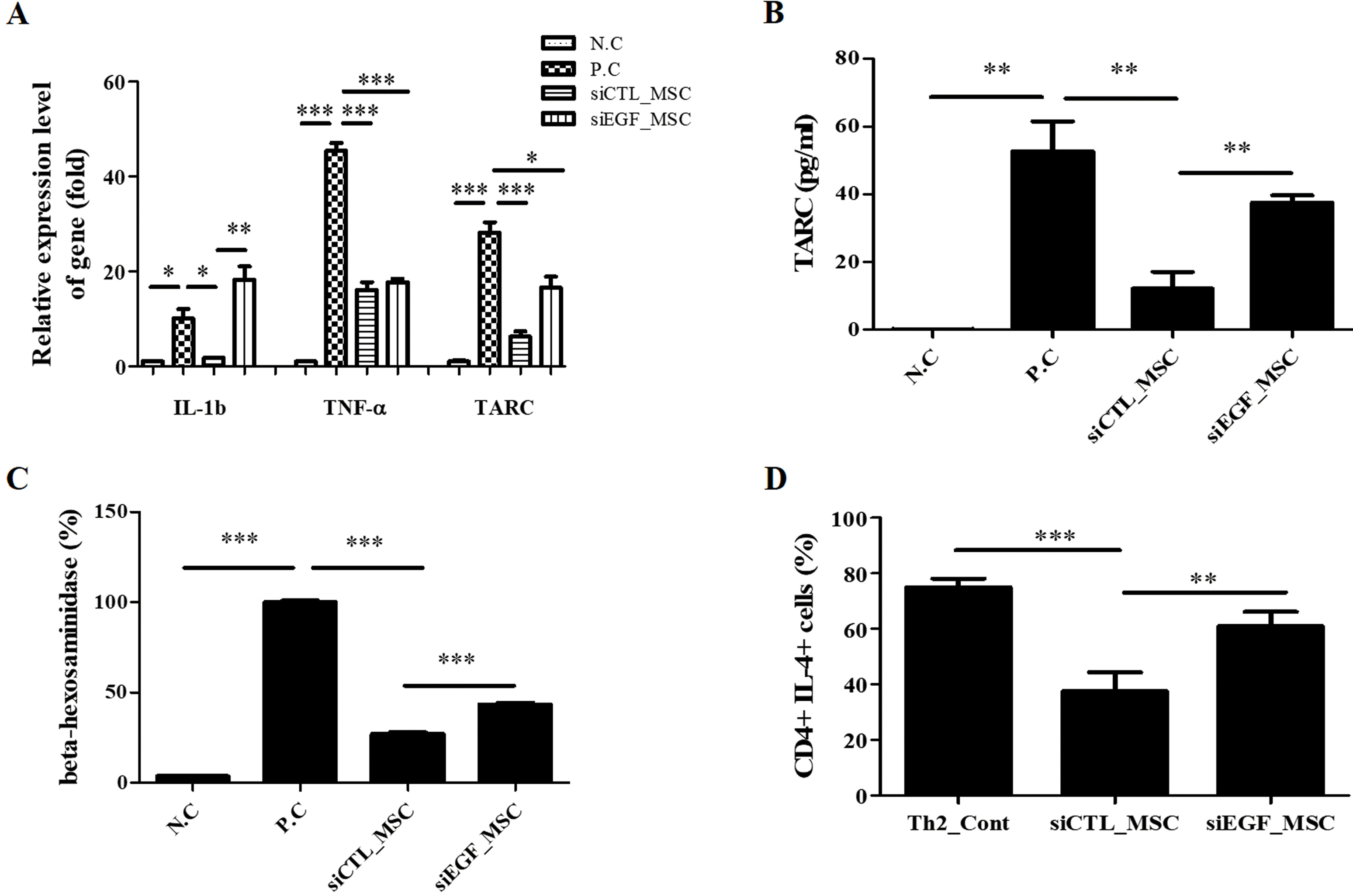
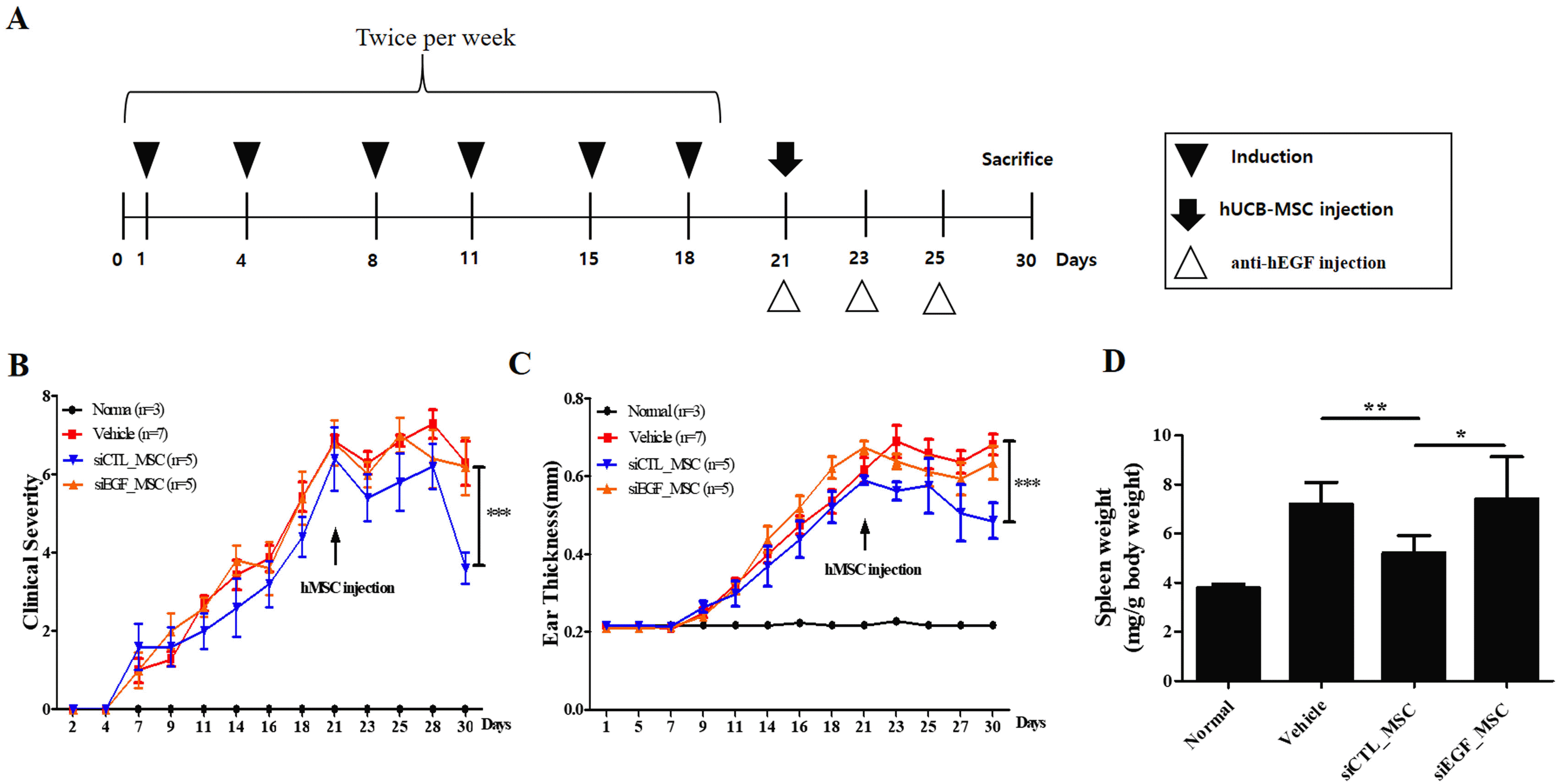




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download