Abstract
Background and Objectives
Currently, safety pharmacological tests for the central nervous system depend on animal behavioral analysis. However, due to the subjectivity of behavioral analysis and differences between species, there is a limit to appropriate nervous system toxicity assessment, therefore a new neurotoxicity assessment that can simulate the human central nervous system is required.
Methods and Results
In our study, we developed an in vitro neurotoxicity assessment focusing on neuronal function. To minimize the differences between species and fast screening, hiPSC-derived neurons and a microelectrode array (MEA) that could simultaneously measure the action potentials of the neuronal networks were used. After analyzing the molecular and electrophysiological characters of our neuronal network, we conducted a neurotoxicity assessment on neurotransmitters, neurotoxicants, illicit drugs, and new psychoactive substances (NPS). We found that most substances used in our experiments responded more sensitively to our MEA-based neurotoxicity assessment than to the conventional neurotoxicity assessment. Also, this is the first paper that evaluates various illicit drugs and NPS using MEA-based neurotoxicity assessment using hiPSC-derived neurons.
Neurotoxicity is caused by exposure to natural and artificial substances that can produces an adverse effect on survival or function of neural systems. The range of neurotoxicants is very broad. Representative neurotoxicants include some heavy metals (arsenic, manganese dioxide), pesticides (cyhalothrin, ivermectin, fenvalerate), pharmaceuticals (naloxone, naltrexone, propranolol), certain gases (carbon monoxide, carbon disulfide), and volatiles (xylenes, trichloroethylene) (1). Currently, about 80,000 chemicals are registered in the EPA and distributed to the market. However, only about 200 are confirmed to be neurotoxic to humans (2). More than 60,000 commercial chemicals have existed in the market without safety evaluation (3).
To evaluate neurotoxicity, in vivo studies with rodents are used according to the TG424 regulation defined by the OECD (Organisation for Economic Co-operation and Development) (4). Despite the advantages of animal experiment, they are time-consuming and labor-intensive. In addition, the cost for evaluating or testing numerous chemicals added annually is high. Differences between rodent species may also cause errors in the interpretation of experimental results. Moreover, there are many ethical problems related to the use of rodents. Accordingly, there is a need for an in vitro assessment method that can reduce or replace animal experiments and test many substances with relatively little labor (1).
There are many in vitro neurotoxicity assessments. To assess neurotoxicity, reduced cell viability, damaged organelle, changed apoptosis-related gene expression level, and reactive oxygen species production are generally measured after treating cells such as human tumor cells, animal primary cells, and human primary cells with target substances (5-7). The method of measuring cell viability has been used for a long time as a common method. However, the sensitivity of these methods is poor to substances that can damage electrophysiological function by blocking metabotropic receptors or ion channels (ex., tetrodotoxin) (8-10). This is because even if there is a damage in the functional aspect, it does not immediately lead to a change in cell viability.
Despite many neurotoxicity assessment studies, neurotoxicity assessment that focuses on neuronal function has not been fully established yet. In our study, an instrument called microelectrode array (MEA) was used to measure neuronal function. Unlike the patch-clamp technology that measures intracellular space electrophysiology by piercing a portion of the membrane of a single cell, MEA uses a micro-electrode inserted in a culture dish to non-invasively and simultaneously measure electrophysiological activities of numerous neurons with relatively less labor and cost (11). Of course, the neurotoxicity of several substances, such as novel psychoactive substances, chemicals, toxins, and drugs, has already been tested using MEAs with neurons derived from mice or brain slices (11-14). However, the MEA evaluation method using human-derived neurons without species differences has great significance as the next-generation neurotoxicity for a human safety test (15, 16).
In this paper, we established an in vitro neurotoxicity assessment using MEA with hiPSC-derived neurons focusing on neuronal function. First of all, we performed basic molecular and functional characterization for hiPSC-derived neurons. Next, we performed in vitro neurotoxicity assessments for diverse substances by using established MEA-based neurotoxicity assessment. Representative neurotransmitter (L-glutamic acid, GABA, epinephrine, dopamine, serotonin, and acetylcholine) and neurotoxicant (D-AP5, CNQX, bicuculline, tetrodotoxin, 4-aminopyridine and tetraethylammonium) were tested. Typical illicit drugs (methamphetamine, cocaine, THC) and new psychoactive substances (3,4-Dichloromethylphenidate, LY-2183240, 4-EA-NBOMe, AB-CHFURYCA) were also tested. For comparison MEA-based neurotoxicity assessment to conventional neurotoxicity assessment, MTT assay was performed for same substances. Most substances tested in in vitro neurotoxicity assessments showed lower IC50 values based on MEA than those based on cell viability.
Taken together, our study is the first report to apply hiPSC-derived neurons to diverse NPS. Also, we highlight the differences between the neurotoxicity assay based on cell viability and MEA through direct comparisons.
L-Glutamic acid, acetylcholine chloride, serotonin hydrochloride, (−)-epinephrine, dopamine hydrochloride and tetraethylammonium chloride were purchased from sigma-aldrich. Other chemicals such as GABA, D-AP5, CNQX, bicuculline, tetrodotoxin and 4-aminopyridine were purchased from tocris bioscience. In case of illicit drugs like methamphetamine, cocaine, tetrahydrocannabinol, 3,4-dichloromethylphenidate, LY-2183240, 4-EA-NBOMe and AB-CHFURYCA (purity >98.5%) were obtained from Ministry of Food and Drug Safety (Cheongju, Republic of Korea). NeurosightⓇ-S EP Maintenance (EPM) Media and NeurosightⓇ-S Plating Maintenance Media (PMM) were purchased from NEXEL corporation. Stock solutions of chemicals and drugs were prepared as each manual on the day of the experiments. Used chemicals were listed on Table 1.
Used chemicals
hiPSC-derived neurons, Neurosight-S (N-002, Lot#-88BSFWL) were purchased from NEXEL corporation. For the immunocytochemistry and cell viability assay, frozen vials were thawed in 37℃ water bath for 1 minute and added dropwise to the 9 ml of PMM. Subsequently, cells were centrifuged at 180G for 3 minutes at room temperature, the supernatant was removed and the pellet was resuspended using 1 ml of PMM for counting with a hematocytometer and trypan blue. The cells were plated to each well (33,000 cells/well) of 96 well cell culture plate coated with 10 μg/ml of laminin (Thermo Fisher, USA) and poly-l-ornithine (Sigma, USA). The day after plating the cells, the half of the media was changed to fresh PMM every two days and the cells were maintained at 37℃ in a humidified atmosphere containing 5% CO2 until the experiments. In case of the microelectrode array assay, cells were thawed as described above, but resuspended using PMM to match the plating density (5 μl per 50,000 cells). After gentle pipetting, laminin was added to a final concentration of 10 μg/ml. As a Next, the cells were plated on a 24 wells MEA plate (Axion Biosystems Inc, Atlanta, UA) coated with poly-l-ornithine. The cells were only placed to the center of the wells in a manner that forms a drop over the electrodes and placed at 37℃ in a humidified atmosphere containing 5% CO2 for 30 minutes for attachment. After then, 500 μl of PMM (5×106 cells) containing 1 μg/ml laminin was gently added to each of the wells. The day after plating the cells, the half of media was changed to fresh EPM media containing 1 μg/ml of laminin every two days, and the cells were maintained at 37℃ in a humidified atmosphere containing 5% CO2 until the experiments.
Two weeks after plating, each of the substances was treated for 24 hours. After removing all substances, 5 mg/ml of MTT solution (Sigma) were diluted 10 times by PMM, added in 100 μl to each well, and incubated in a CO2 incubator in the dark for 1 hour. After then, the solution was removed and formed formazan crystals were dissolved using 50 μl of DMSO for each the well. The absorbance was read at 570 nm using a fluorescence microplate reader, Gemini EM (Molecular Devices, USA). The average values and IC50 values were statistically analyzed using non-linear regression by GraphPad prism (ver 7.00, USA). Also, for determine the significant concentration range, a one-way ANOVA followed by a post-hoc Dunnett’s test was used. p<0.05 compared to the control showed*.
The hiPSC-derived neurons were cultured for 4 weeks for the experiments. The whole process was conducted considering other previous studies (11, 13, 15). Briefly, for recording spontaneous neuronal activity, MEA-24 well plates (M384-tMEA-24W) containing 16 microelectrodes in each well were used with Axion Maestro (Axion BioSystem Inc, USA). Before the recording, all media were fully changed to 500 μl of fresh media and stabilized in 37℃, 5% CO2 condition for 1 hour. After then, MEA-24 well plates were placed in axion maestro conditioned 37℃, 5% CO2 for equilibration during 2∼3 minutes. After then, spontaneous neural activity was recorded for 30 minutes as the baseline. Based on baseline recording, the well which has more than one active electrode (>0.1 spikes/sec) were selected for the experiments. Prior to treatment of substances, 100 μl of media was removed and all substances prepared as 100 μl of 5X concentrates were treated by manually pipetting. After treatment, spontaneous neuronal activity was measured for 30 minutes to identify the effect of substances. For deciding the concentration range for MEA assay, cell viability assay by MTT assay was preceded. Based on the LC50 values in the MTT assay, the treatment range was determined up to the concentration at which the drug did not show any change in MEA. Also, all of each well were used for only one substance and one concentration for avoiding cumulative dosing and receptor desensitization. For one experimental condition, a total of 10 to 12 repetitions were performed using different wells in 5∼6 plates.
The whole process was conducted considering other previous studies (11, 13, 15). Briefly, after recording spontaneous neuronal activity, all raw data files were analyzed by using Axis Navigator (2.0.4.21ver, USA) for quantifica-tion. Spikes were defined as >7xSD of the internal noise level (rms) with a post/pre spike duration of 3.6/2.4 ms of each electrode. For measuring reaction to the neurotransmitter, 10 minutes of the initial part in raw data were analyzed after removing first 2 minutes while the end of 10 minutes in raw data was analyzed for the neurotoxicity assessment to neurotransmitter, neurotoxicant and illicit drug. Out of indices, weighted mean firing rate (wMFR) was used for deciding the effect of all substances. After obtaining wMFR values, the ratio of the baseline wMFR and the treatment wMFR was calculated and expressed a percentage (Treatment ratio). Also, the treatment ratio ≥average±2SD was considered as an outlier and excluded. Calculated treatment ratios were analyzed by GraphPad prism software. Concentration values were transferred to log values and non-linear regression was used for calculating IC50 values. To find significant differences depend on concentration, a one-way ANOVA followed by a post-hoc Dunnett’s test was used by GraphPad Prism software. Compare to wMFR values of baseline and wMFR values of drug treated wells, we found the changed ratio depend on concentration. Also, Increasing or decreasing of wMFR was considered as significant when the effect was statistically significant (p<0.001) and ≥30%.
The cells were fixed with 4% paraformaldehyde (Bio-sesang, Republic of Korea) for 30 minutes at room temperature and washed two times with DPBS (Welgenes). Next, blocking solution containing 0.1% triton X-100 (Promega, USA) and 5% FBS (ThermoFisher) diluted with DPBS were treated for 1 hour at room temperature. The cells were incubated with primary antibodies for 16 hours at 4℃, washed two times with DPBS, and finally incubated with appropriate fluorescence-conjugated secondary antibodies for 1 hour at room temperature without light. Nucleic were stained with Hoechst 33342 (Thermo-Fisher, USA). After the staining, Three ICC images were captured per batch by fluorescent microscope with 20× magnification. Each cells were manually counted from obtaining images by image J software and repeated three times. Averaged number of vGlut+ cells or GABA+ cells were divided by total number of DAPI+ cells for detecting the ratio of glutamatergic neurons and GABAergic neurons.
The antibodies used in our research for immunocy-tochemistry: Anti-Vesicular Glutamate Transporter 2 Antibody (MAB5504, Merck, 1 : 300, mouse), Anti-NeuN antibody (ab128886, Abcam, 1 : 200, rabbit), Synapsin 1 antibody (106 011, Synaptic Systems, 1 : 100, mouse), Anti-Neurofilament 200 antibody (N4142, Merck, 1 : 500, rabbit) and Anti-GABA antibody (A2052, Sigma, 1 : 200, rabbit) as primary antibodies. For secondary antibodies, Goat anti-Mouse IgG (H+L) Cross-Adsorbed Secondary Anti-body, Alexa Fluor 488 (A-11001, Invitrogen, 1 : 1000, goat) and Goat anti-Rabbit IgG (H+L) Cross-Adsorbed Secon-dary Antibody, Alexa Fluor 594 (A11012, Invitrogen, 1 : 1000, goat) were used.
Neuronal gene expression levels were evaluated by qPCR, using GAPDH gene as internal standard. To extract RNA, Trizol reagent (ThermoFisher, USA) was used. Total RNA was reversetranscribed into complementary DNA (cDNA) using cDNA synthesis kit (ThermoFisher, USA) following the manufacture’s protocol. PCR reaction was carried out using SYBRⓇ Green Quantitative RT-qPCR Kit (Roche, Switzerland) and a Roche real-time PCR system (LightCyclerⓇ 96). ΔCt values were calculated by subtracting the GAPDH Ct value from that of target genes. Relative expression levels were calculated using the 2−ΔΔCt method. Primer sequences and the size of amplicons are listed in Table 2.
Primer sequences and the size of amplicons
Molecular analysis: To perform the basic characterization of hiPSC-derived neurons to be used for neurotoxicity assessment, hiPSC-derived neurons were cultured and analyzed at the molecular level at different time points. These cultured cells showed dendrites as a typical neuron structure at around day 10 after seeding. Branched structure between cells was denser at day 20 compared to its day 10 (Supplementary Fig. S1A). Next, expression levels of neuronal markers in neurons such as NEUROFILAMENT-H and NEUN were determined by ICC at around day 20. Synapse formation was also examined by ICC through the expression level of synapse marker SYNAPSIN 1. GABA was also evaluated by ICC in some cells (Supplementary Fig. S1B). Results confirmed that more than 90% of cells were vGlut2+ glutamatergic neurons, while less than 3% of cells were GABA+ GABAergic neurons (Supplementary Fig. S1B and S1C). Furthermore, expression levels of typical neuronal markers such as TUBB3, NF, SLC1717, SLC1716, and MAP2 were determined by qPCR (Supplementary Fig. S1D).
Electrophysiological analysis and response to neurotransmitters: Next, changes of electrical physiology in hiPSC-derived neurons by week were analyzed. When neurons mature and have electrophysiological properties, they transmit signals through changes in cell membrane potential. The MEA detects the extracellular action potential changes of neurons cultured on electrodes and converts the potential changes which exceed specific set value as spikes. wMFR represents overall cell population excitability and connectivity by averaged spike amplitude over active electrodes. Number of burst and burst duration represent quantifying connectivity through neural network (17). Cultured hiPSC-derived neurons’ wMFR, number of burst, and burst duration were increased by week (Supplementary Fig. S2A). Network bursts were confirmed from the 3rd weeks after culture. The number of network bursts, network burst frequency, and synchrony index were also increased rapidly from the 3rd week following network bursts (Supplementary Fig. S2A, S2C and S2D). The number of active electrodes increased from 2.05±0.97 to 13.3±0.9 after 4 weeks. In the last 4 weeks, an average of 83% of electrodes were active in one well (Supplementary Fig. S2B). Considering the overall experimental arrangement, the MEA plate between the 3rd week and the 4th week after seeding was used for the experiment.
Also, prior to neurotoxicity assessment, we confirmed the presence and functionality of neurotransmitter receptors in our cell culture by treating several neurotransmitter and investigating transient effect to wMFR. For detecting response to neurotransmitter, first 5 minutes of exposure data were analyzed compared to control wells. In case of L-glutamic acid, wMFR was increased to 136±21.1% compared with baseline (100%) at 3 μM, reaching 160.8±9.9%, 239±53.8%, and 298.5±63.9% at 10 μM, 30 μM, and 100 μM, respectively. From 300 μM, wMFR of L-glutamic acid was decreased to be under 70%. Dopamine and epinephrine also showed increasing wMFRs up to 122±3.6 and 183±28.7% at 0.3 μM and 10 μM, respectively. However, at 100 μM, wMFRs were decreased to 62±4.5%, indicating that the range of the dopamine and epinephrine concentration is for cell death. In case of acetylcholine, GABA, and serotonin, they all showed decreased wMFRs (66±14.3% for acetylcholine, 44±20% for GABA, and 57±11.3% for serotonin) at 10 μM (Supplementary Fig. S2E, Table S1). Taken together, these results showed the molecular and electrophysiolo-gical maturation of cultured hiPSC-derived neurons. Also, we confirmed that hiPSC-derived neurons in our systems reacted differently according to neurotransmitters.
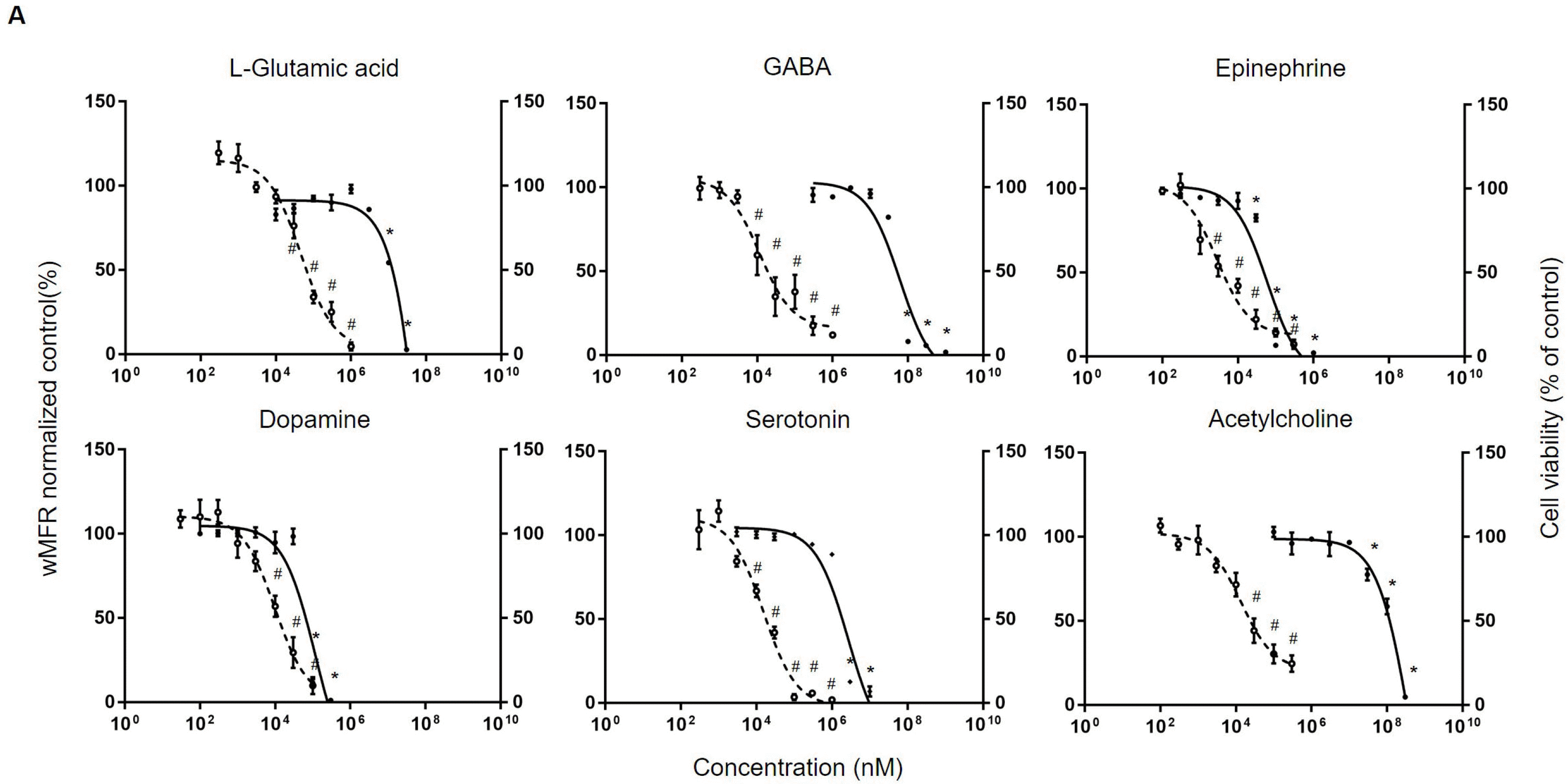
Neurotransmitters: First, neurotransmitters were tested by both neurotoxicity assays and compared: according to previous study, overdose of neurotransmitter can also act as a neurotoxicant. Before assessments, hiPSC-derived neurons were cultured for about 21∼28 days. The concentration applied to MTT assay was determined considering previous findings (11, 18-25). In the MTT assay, L-glutamic acid, a representative neurotransmitter of excitatory neurons, did not significantly affect the viability of hiPSC-derived neurons at concentration up to 3 mM. Its IC50 was 157.6 mM. GABA, a representative neurotrans-mitter of inhibitory neurons, did not affect the viability of hiPSC-derived neurons at concentration up to 10 mM. Its IC50 was 60.6 mM. Monoamine neurotransmitters epinephrine, dopamine, serotonin, and acetylcholine did not show toxicity at concentrations up to 30 μM, 30 μM, 1 mM, and 10 mM, with IC50 values of 59 μM, 118.7 μM, 2.7 mM, and 426.5 mM, respectively.
Electrophysiological changes regarding various neurotransmitters in hiPSC-derived neurons were then measured. L-glutamic acid decreased wMFR from concentration of 100 μM (to 33±6.5%). Treatment with 10 μM of GABA decreased wMFR to 59.6±20.6%. Treatments with 30 μM and 300 μM of GABA also decreased wMFR to 34.9±20% and 17.5±9.6%, respectively, following previous trends. IC50 values of L-glutamic acid and GABA were 46.6 μM and 11.5 μM, respectively. In case of dopamine, one of neurotransmitter in monoamine group, it decreased wMFR to 56±6.3% at 10 μM. Its IC50 value was 9.8 μM. Other monoamine group neurotransmitters epinephrine, serotonin, and acetylcholine also decreased wMFR. In detail, 100 μM of epinephrine decreased wMFR to 14.1±4.2%. Serotonin at 10 μM and 100 μM decreased wMFR to 66.8±6.5% and 3.4±3.1%, respectively. Acetylcholine also decreased wMFR to 44.2±12.5% at 30 μM. IC50 values of epinephrine, serotonin, and acetylcholine were 2.8 μM, 15.4 μM, and 13.5 μM, respectively (Fig. 1A).
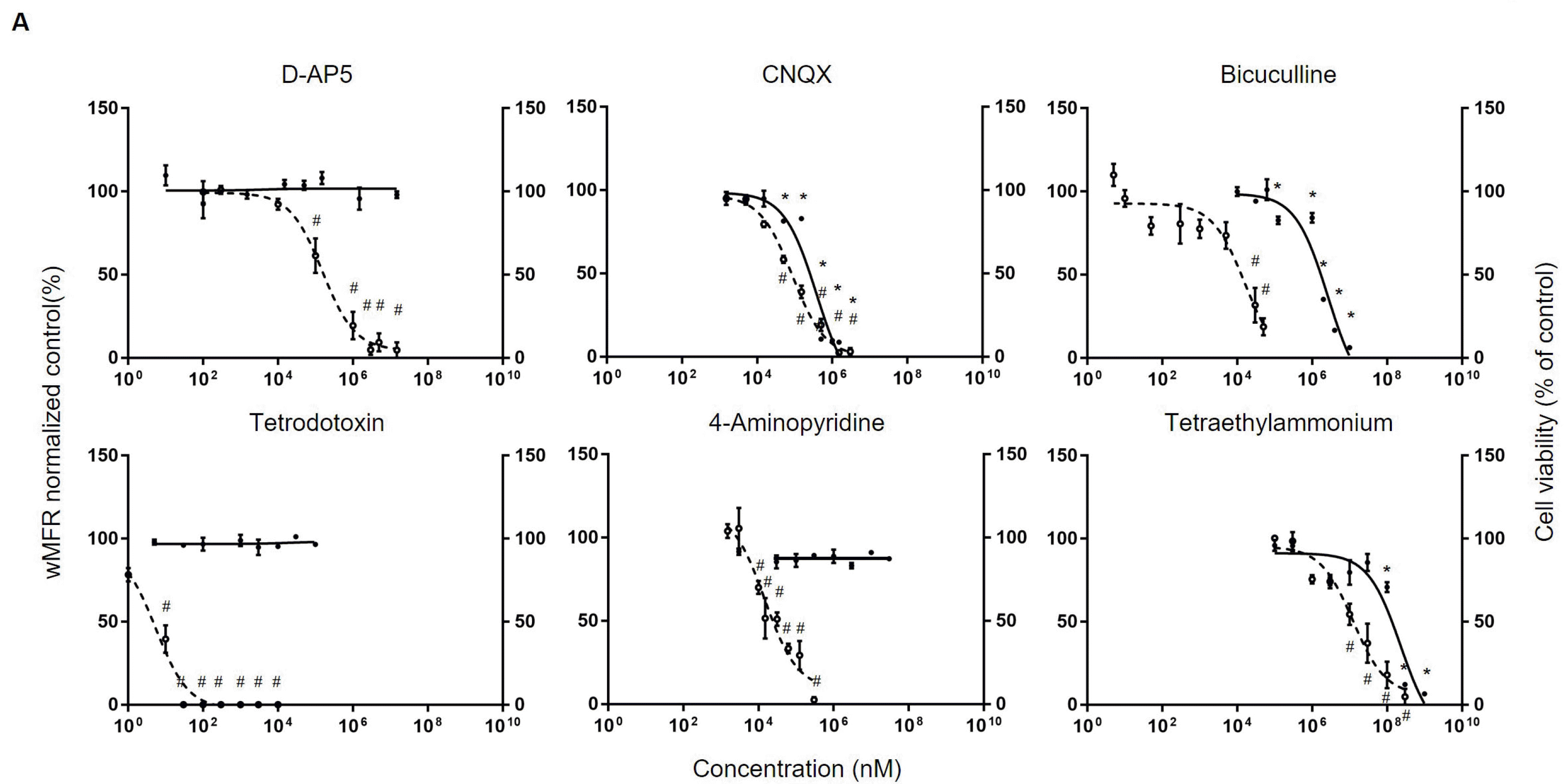
Neurotoxicants: Next, using representative neurotoxicant, neurotoxic assessment was performed using MTT assay or MEA. In the case of DAP5, a representative glutamatergic receptor NMDAR antagonist, there was no significant change in cell viability even at the maximum solubility of 15 mM. However, CNQX, an antagonist of AMPAR, decreased cell viability from concentration of 100 μM. Its IC50 was 383.6 μM. Bicuculline, an antagonist of GABA A receptor, significantly decreased cell viability at concentration from 125 μM. Its IC50 was 2.6 mM. However, tetrodotoxin, a representative sodium channel blocker, and 4-aminopyridine, a potassium channel blocker, did not significantly affect cell viability at concentration up to 100 μM and 30 mM, respectively. In the case of tetraethylammonium, another potassium channel blocker, a significant decrease in cell viability was observed at 100 mM. Its IC50 was 230.6 mM (Fig. 2A).
Electrophysiological changes were confirmed through wMFR by MEA array as shown in previous results. In the case of D-AP5, a significant decrease in wMFR occurred from concentration of 100 μM (wMFR decreased to 61.4±23.2%). Its IC50 was 153.9 μM. Similarly, in the case of CNQX, the wMFR decreased significantly (to 58.4±3.8%) from concentration of 50 μM. Its IC50 was 87.1 μM. Bicuculline showed a significant decrease of wMFR (to 31.7±17.9%) from concentration of 30 μM. Its IC50 was 15.9 μM. Tetrodotoxin showed no spikes from 0.03 μM. Its IC50 was 5.5 nM. Both 4-aminopyridine at 10 μM and tetraethylammonium at 10 mM showed significant decreases in wMFR to 70±6.6% and 54.4±14.2%, respectively. Their IC50 values were 14.7 μM and 12.4 mM, respectively (Fig. 2B). Overall, in case of neurotoxic substances, hiPSC-derived neurons showed a rapid decline in electrophysiological function even at concentrations that did not significantly change cell viability.
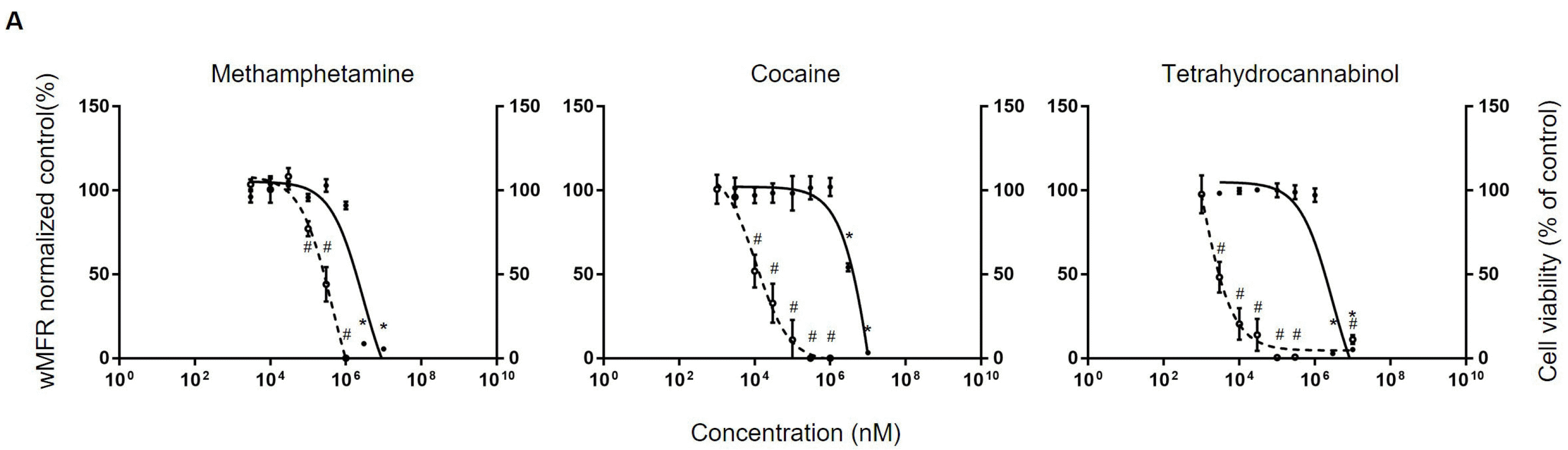
Typical illicit drugs: After testing neurotoxicants, we tried to determine whether hiPSC-derived neurons could be used to evaluate neurotoxicities of illicit drugs. Firstly, representative illicit drugs were tested through MTT assay and MEA. As classic illicit drugs, methamphetamine, cocaine, and tetrahydrocannabinol (THC) were used. For the MTT assay, the concentration was set based on previous findings (26-28). In case of methamphetamine, it induced a significant decrease in cell viability from 3 mM. Cocaine and THC also decreased cell viability from 3 mM. IC50 values of methamphetamine, cocaine, and THC were 2.9 mM, 18.1 mM, and 2.9 mM, respectively. After that, electrophysiological changes according to different concentrations were determined through MEA array. Metham-phetamine resulted in a significant decrease in wMFR (44.1±17.8%) from 300 μM. Its IC50 value confirmed by MEA array was 300.5 μM. In the case of cocaine, it was found significantly decreased wMFR (to 52±9.8%) from concentration of 10 μM. Its IC50 was 11 μM. THC also significantly decreased wMFR (to 48.3±18.4%) from concentration of 30 μM. Its IC50 value was found to be 8.1μM (Fig. 3A).
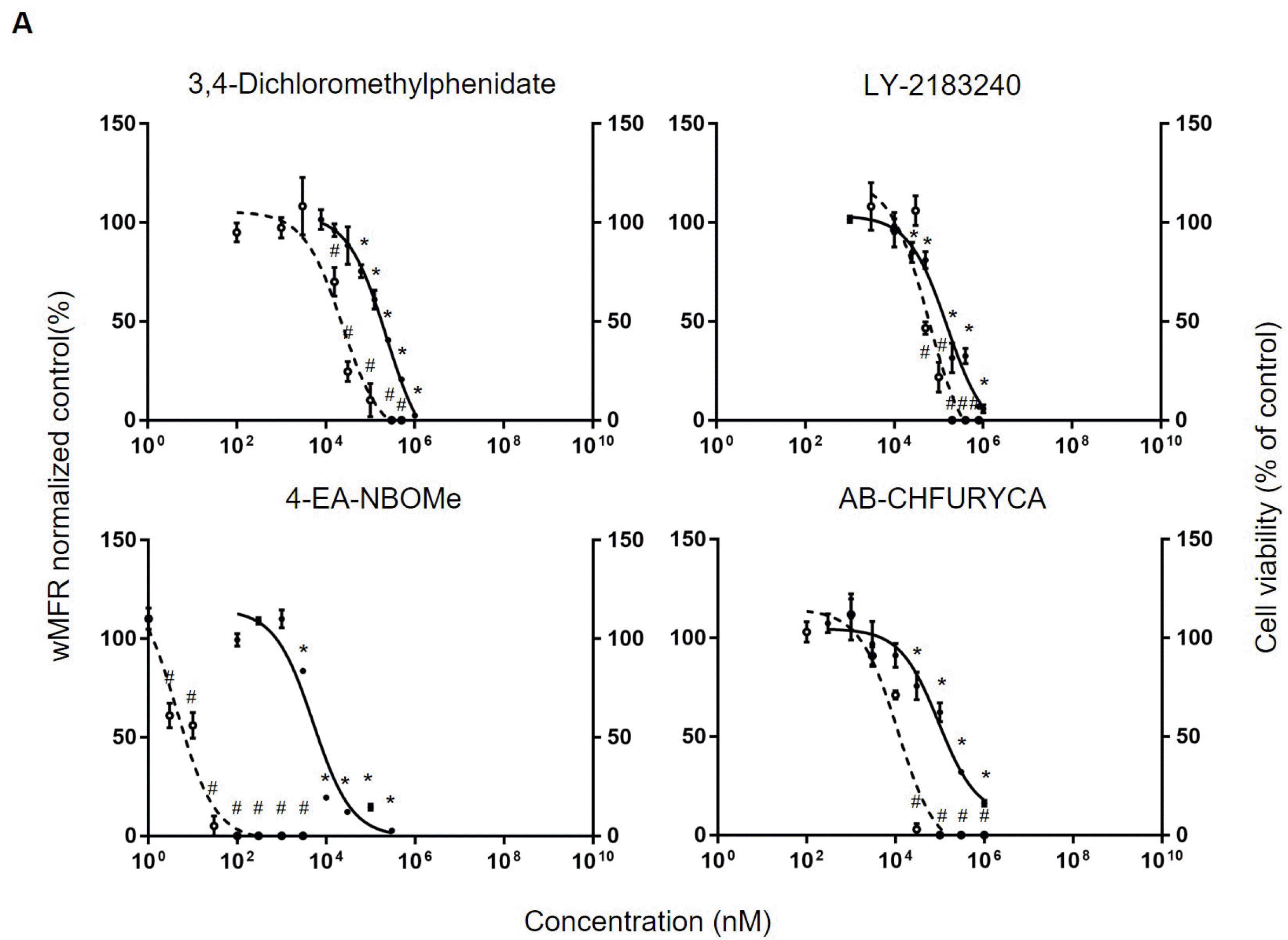
New psychoactive substances (NPSs): Based on response of hiPSC-derived neurons to classic illicit drugs, we conducted a neurotoxicity assessment for our NPSs. In this study, 3,4-dichloromethylphenidate (3,4-DCMP), 4-EA-NBOMe, LY-2183240, and AB-CHFUPYCA were used as NPSs. The maximum solubility and experimental concentration were set based on previous findings (29-32). As a result of determining effects of NPS at different concentrations on cell viability using MTT assay, 3,4-DCMP caused a significant decrease in cell viability from 6.25 μM. Its IC50 was found to be 230.6 μM. LY-2183240, 4-EA-NBOMe, and AB-CHFURYCA also significantly decrea-sed cell viability from 50 μM, 3 μM, and 30 μM, res-pectively. IC50 values of LY-2183240, 4-EA-NBOMe, and AB-CHFURYCA were 153.7 μM, 5.1 μM, and 93.6 μM. As a next, electrophysiological changes were confirmed by MEA array. 3,4-DCMP did not result in a significant decrease in wMFR until 3 μM. However, at 15.6 μM and 100 μM, it resulted in sharp decreases of wMFR to 66.7±8.0% and 10.2±14.5%, respectively. Its IC50 based on MEA array was 23.5 μM. LY-2183240 resulted in a significant decrease in wMFR (to 46.7±5.4%) from concentration of 50 μM. Its IC50 was 59.2 μM. 4-Ea-NBOMe from 3 nM and AB-CHFURYCA from 30 μM resulted in sharp decreases in wMFR (to 61.1±11.0% and 2.9±5.0%, respectively). IC50 values of 4-Ea-NBOMe and AB-CHFU RYCA were 4.3 nM and 11.3 μM, respectively (Fig. 4A). Overall, hiPSC-derived neurons showed rapid electrophysiological changes in concentrations that did not significantly change cell viability even in drug-related substances.
MEA have been used for about 10 years for neurotoxicity evaluation. The neurotoxicity of the seizurogenic compound (33), illicit drug, NPS (11, 13, 34), pyrethroids (35), and anticancer drugs (12) were evaluated by MEA. Most studies applied rat primary neurons mixed with astrocytes. Rat primary neurons were highly functional and easy to take from animal. However, there still has doubt whether this result could fully reflect human cases. Recently, several papers applied hiPSC-derived neurons with MEA for neurotoxicity assay, but the number of tested substances was still quite limited (12, 15, 33, 36-38). Especially, the cases which tested NPS using hiPSC-derived neurons were few (37). Here, we evaluated the neurotoxicity of the representative neurotransmitter, neurotoxicants, and NPSs using hiPSC-derived neurons and MEA and compared it with the results evaluated by the MTT assay.
The hiPSC-derive neurons consisted of 90% of glutamatergic neurons and less than 3% of GABAergic neurons. Although it didn’t accurately simulate the ratio of in vivo (7 : 3), it reacts to the treatment of GABA with decreasing wMFR. This showed that the portion of GABAergic neurons less than 3% could affect the overall neuronal activity. Lu et al. (39) also showed similar results. In Supplementary Fig. S1E, the hiPSC-derived neuron reacts concentration-dependently after exposure to six neurotransmitters. Unlike Fig. 1 which evaluated the neurotoxicity of neurotransmitters, Supplementary Fig. S1E showed the response following the 5 minutes of exposure to neurotransmitters. The representative excitatory neurotransmitter, L-glutamic acid, dopamine, and epinephrine increased neuronal activity depend on the concentration. However, at high concentrations, they almost inhibit neuronal activity. This, inhibition of neuronal activity at a high concentration of excitatory neurotransmitters was also observed in other studies which used rat primary neurons with MEA for neurotoxicity evaluation (11). The inhibitory neurotransmitter, GABA, acetylcholine, and serotonin decreased neuronal activity concentration-dependently. Taken together, these results showed that the hiPSC-derived neuron has a similar electrophysiological character with rat primary neurons.
The hiPSC-derived neuron showed active network burst from 3rd weeks following seeding. Considering previous studies (11-14), we used the hiPSC-derive neurons cultured for more than three weeks. The IC50 evaluated by MEA or MTT was listed in Table 3. The comparison value showed the quantified differences between the result of MEA and MTT assay. It is well known that the neurotoxicity assay using MEA is highly sensitive compared with the MTT assay, however, no study showed quantitative comparison. Excluding the substances which couldn’t get IC50 by MTT assay, the IC50 values get by MTT assay showed 102 times higher more than the IC50 values get by MEA. As shown in Fig. 1 and 2, neurotransmitters or neurotoxicants including ion channel-related drugs showed a more sensitive response in MEA assay than MTT. Even though the difference in IC50 concentration seemed small in the graph (dopamine and CNQX), the numerical concentration difference was 3 to 5 times or more. This difference is caused by the following mechanism of drug response. MEA is a reaction of a substance that directly affects nerve excitation (receptors, ion channels), and MTT is a secondary result of neuron overexcitation or intracellular organelles such as mitochondria.
IC50 (MTT and MEA)
NPS is a psychotropic substance made by modifying some chemical formulas of existing illicit drugs. It is estimated that there are about 1,000 NPS currently existing in the world (40, 41). Because dozens of NPS are newly identified every year, this field requires rapid development of toxicity assessment. However, it is not easy to handle illicit drugs for research, so only a few studies existed. We legally received several illicit drugs from the Korean Ministry of Food and Drug Safety and conducted the neurotoxicity evaluation. For comparison, we found other studies that applied the same drugs we tested. In the case of cocaine, their IC50 was 9.8 μM or 10 μM similar to our IC50 (11 μM). Interestingly, the IC50 of methamphetamine from other studies was 100 μM or 116 μM, while our values were 300.5 μM (13, 34).
There are several limitations to this paper. Firstly, because our commercial products don’t include astrocytes, we only used neurons. Considering that some recent studies used hiPSC-derived neurons mixed with astrocytes for MEA based neurotoxicity evaluation (33, 36, 38), we also need to apply the cells similar with in vivo. Especially, astrocytes have essential functions in neuronal survival. The IC50 differences of methamphetamine between other studies could be caused by the presence of astrocytes. Secondly, because we couldn’t select the illicit drug or NPS to be tested, the comparison based on each of the chemical structures was difficult. If we could choose, we would be able to select analogs based on their chemical structures and find the effect on IC50 values by specific structure. We expect that more analogs studies will be accumulated to further understanding the linkage between the chemical structure and IC50.
Nevertheless, we evaluated the neurotoxicity of new substances that had not been tested before using hiPSC-derived neurons. Accumulation of these data will help to make a database for alternative neurotoxicity assay in the future. In addition, we quantitatively confirmed the differences between MEA based neurotoxicity assay or MTT based neurotoxicity assay. This result will be meaningful for the researchers who must select a cell-based neurotoxicity assay.
Supplementary data including one table and two figures can be found with this article online at https://doi.org/10.15283/ijsc21217.
Acknowledgments
This work was supported by a grant 20182MFDS423 and 19182MFDS408 from Ministry of Food and Drug Safety in 2021.
References
1. Bal-Price A, Hogberg HT, Crofton KM, Daneshian M, FitzGerald RE, Fritsche E, Heinonen T, Hougaard Bennekou S, Klima S, Piersma AH, Sachana M, Shafer TJ, Terron A, Monnet-Tschudi F, Viviani B, Waldmann T, Westerink RHS, Wilks MF, Witters H, Zurich MG, Leist M. 2018; Recommendation on test readiness criteria for new approach methods in toxicology: exemplified for developmental neurotoxicity. ALTEX. 35:306–352. Erratum in: ALTEX 2019;36:506. DOI: 10.14573/altex.1904112. PMID: 31329255. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85070184812&origin=inward.
2. Grandjean P, Landrigan PJ. 2006; Developmental neurotoxicity of industrial chemicals. Lancet. 368:2167–2178. DOI: 10.1016/S0140-6736(06)69665-7. PMID: 17174709. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33845424655&origin=inward.
3. Scialla M. 2016. Jun. 22. It Could Take Centuries for EPA to Test All the Unregulated Chemicals Under a New Landmark Bill [Internet]. PBS News Hour;Arlington: Available from: https://www.pbs.org/newshour/science/it-could-take-centuries-for-epa-to-test-all-the-unregulated-chemicals-under-a-new-landmark-bill. cited 2021 Nov 1.
4. Bal-Price A, Hogberg HT, Crofton KM, Daneshian M, FitzGerald RE, Fritsche E, Heinonen T, Hougaard Bennekou S, Klima S, Piersma AH, Sachana M, Shafer TJ, Terron A, Monnet-Tschudi F, Viviani B, Waldmann T, Westerink RHS, Wilks MF, Witters H, Zurich MG, Leist M. 2019; Corrigendum to recommendation on test readiness criteria for new approach methods in toxicology: exemplified for developmental neurotoxicity. ALTEX. 36:506. Erratum for: ALTEX 2018;35:306-352. DOI: 10.14573/altex.1904112. PMID: 31329255. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85070184812&origin=inward.
5. Tong ZB, Hogberg H, Kuo D, Sakamuru S, Xia M, Smirnova L, Hartung T, Gerhold D. 2017; Characterization of three human cell line models for high-throughput neuronal cytotoxicity screening. J Appl Toxicol. 37:167–180. DOI: 10.1002/jat.3334. PMID: 27143523. PMCID: PMC5094908. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84965130907&origin=inward.
6. Leist M, Jäättelä M. 2001; Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol. 2:589–598. DOI: 10.1038/35085008. PMID: 11483992. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0035433420&origin=inward.
7. Pak YL, Swamy KM, Yoon J. 2015; Recent progress in fluorescent imaging probes. Sensors (Basel). 15:24374–24396. DOI: 10.3390/s150924374. PMID: 26402684. PMCID: PMC4610470. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84942134014&origin=inward.
8. Castoldi AF, Coccini T, Ceccatelli S, Manzo L. 2001; Neuro-toxicity and molecular effects of methylmercury. Brain Res Bull. 55:197–203. DOI: 10.1016/S0361-9230(01)00458-0. PMID: 11470315. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0035872456&origin=inward.
9. Sills RC, Valentine WM, Moser V, Graham DG, Morgan DL. 2000; Characterization of carbon disulfide neurotoxicity in C57BL6 mice: behavioral, morphologic, and molecular effects. Toxicol Pathol. 28:142–148. DOI: 10.1177/019262330002800118. PMID: 10669001. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0033973711&origin=inward.
10. Kiernan MC, Isbister GK, Lin CS, Burke D, Bostock H. 2005; Acute tetrodotoxin-induced neurotoxicity after ingestion of puffer fish. Ann Neurol. 57:339–348. DOI: 10.1002/ana.20395. PMID: 15732107. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=14844318086&origin=inward.
11. Hondebrink L, Verboven AHA, Drega WS, Schmeink S, de Groot MWGDM, van Kleef RGDM, Wijnolts FMJ, de Groot A, Meulenbelt J, Westerink RHS. 2016; Neurotoxicity screening of (illicit) drugs using novel methods for analysis of microelectrode array (MEA) recordings. Neurotoxicology. 55:1–9. DOI: 10.1016/j.neuro.2016.04.020. PMID: 27149913. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84965075992&origin=inward.
12. Wing C, Komatsu M, Delaney SM, Krause M, Wheeler HE, Dolan ME. 2017; Application of stem cell derived neuronal cells to evaluate neurotoxic chemotherapy. Stem Cell Res. 22:79–88. DOI: 10.1016/j.scr.2017.06.006. PMID: 28645005. PMCID: PMC5737666. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85021095824&origin=inward.
13. Zwartsen A, Hondebrink L, Westerink RH. 2018; Neurotoxicity screening of new psychoactive substances (NPS): effects on neuronal activity in rat cortical cultures using microelectrode arrays (MEA). Neurotoxicology. 66:87–97. DOI: 10.1016/j.neuro.2018.03.007. PMID: 29572046. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85044757012&origin=inward.
14. Vassallo A, Chiappalone M, De Camargos Lopes R, Scelfo B, Novellino A, Defranchi E, Palosaari T, Weisschu T, Ramirez T, Martinoia S, Johnstone AFM, Mack CM, Landsiedel R, Whelan M, Bal-Price A, Shafer TJ. 2017; A multi-laboratory evaluation of microelectrode array-based measurements of neural network activity for acute neurotoxicity testing. Neurotoxicology. 60:280–292. DOI: 10.1016/j.neuro.2016.03.019. PMID: 27036093. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84962407428&origin=inward.
15. Tukker AM, Wijnolts FMJ, de Groot A, Westerink RHS. 2018; Human iPSC-derived neuronal models for in vitro neurotoxicity assessment. Neurotoxicology. 67:215–225. DOI: 10.1016/j.neuro.2018.06.007. PMID: 29909083. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85048729038&origin=inward.
16. Park JH, Kim J, Walter J, Kim CY. 2022; Use of neural 3D organoid with MEA in neurotoxicity testing: comparison to traditional in vitro cell culture and in vivo methods. Mol Cell Toxicol. 18:17–21. DOI: 10.1007/s13273-021-00184-z. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85117847297&origin=inward.
17. Suresh J, Radojicic M, Pesce LL, Bhansali A, Wang J, Tryba AK, Marks JD, van Drongelen W. 2016; Network burst activity in hippocampal neuronal cultures: the role of synaptic and intrinsic currents. J Neurophysiol. 115:3073–3089. DOI: 10.1152/jn.00995.2015. PMID: 26984425. PMCID: PMC4946605. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84984804885&origin=inward.
18. Garcia VJ, Rushton DJ, Tom CM, Allen ND, Kemp PJ, Svendsen CN, Mattis VB. 2019; Huntington's disease patient-derived astrocytes display electrophysiological impairments and reduced neuronal support. Front Neurosci. 13:669. DOI: 10.3389/fnins.2019.00669. PMID: 31316341. PMCID: PMC6610155. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85068475175&origin=inward.
19. Mikami Y, Yamazawa T. 2015; Chlorogenic acid, a polyphenol in coffee, protects neurons against glutamate neurotoxicity. Life Sci. 139:69–74. DOI: 10.1016/j.lfs.2015.08.005. PMID: 26285175. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84940048353&origin=inward.
20. Ruehle S, Remmers F, Romo-Parra H, Massa F, Wickert M, Wörtge S, Häring M, Kaiser N, Marsicano G, Pape HC, Lutz B. 2013; Cannabinoid CB1 receptor in dorsal telencephalic glutamatergic neurons: distinctive sufficiency for hippocampus-dependent and amygdala-dependent synaptic and behavioral functions. J Neurosci. 33:10264–10277. DOI: 10.1523/JNEUROSCI.4171-12.2013. PMID: 23785142. PMCID: PMC6618598. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84879135358&origin=inward.
21. Campbell SL, Hablitz JJ, Olsen ML. 2014; Functional changes in glutamate transporters and astrocyte biophysical properties in a rodent model of focal cortical dysplasia. Front Cell Neurosci. 8:425. DOI: 10.3389/fncel.2014.00425. PMID: 25565960. PMCID: PMC4269128. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84919433348&origin=inward.
22. Camp AJ, Lim R, Anderson WB, Schofield PR, Callister RJ, Brichta AM. 2010; Attenuated glycine receptor function reduces excitability of mouse medial vestibular nucleus neurons. Neuroscience. 170:348–360. DOI: 10.1016/j.neuroscience.2010.06.040. PMID: 20600650. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=77955920870&origin=inward.
23. Hanganu IL, Kilb W, Luhmann HJ. 2001; Spontaneous synaptic activity of subplate neurons in neonatal rat somatosensory cortex. Cereb Cortex. 11:400–410. DOI: 10.1093/cercor/11.5.400. PMID: 11313292. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0035011515&origin=inward.
24. Naujock M, Stanslowsky N, Bufler S, Naumann M, Reinhardt P, Sterneckert J, Kefalakes E, Kassebaum C, Bursch F, Lojewski X, Storch A, Frickenhaus M, Boeckers TM, Putz S, Demestre M, Liebau S, Klingenstein M, Ludolph AC, Dengler R, Kim KS, Hermann A, Wegner F, Petri S. 2016; 4-aminopyridine induced activity rescues hypoexcitable motor neurons from amyotrophic lateral sclerosis patient-derived induced pluripotent stem cells. Stem Cells. 34:1563–1575. DOI: 10.1002/stem.2354. PMID: 26946488. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84973109229&origin=inward.
25. Stotz SC, Scott LO, Drummond-Main C, Avchalumov Y, Girotto F, Davidsen J, Gómez-Gárcia MR, Rho JM, Pavlov EV, Colicos MA. 2014; Inorganic polyphosphate regulates neuronal excitability through modulation of voltage-gated channels. Mol Brain. 7:42. DOI: 10.1186/1756-6606-7-42. PMID: 24886461. PMCID: PMC4061113. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84902833632&origin=inward.
26. Gilbert GL, Kim HJ, Waataja JJ, Thayer SA. 2007; Delta9-tetrahydrocannabinol protects hippocampal neurons from excitotoxicity. Brain Res. 1128:61–69. DOI: 10.1016/j.brainres.2006.03.011. PMID: 17140550. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33846066404&origin=inward.
27. Poon HF, Abdullah L, Mullan MA, Mullan MJ, Crawford FC. 2007; Cocaine-induced oxidative stress precedes cell death in human neuronal progenitor cells. Neurochem Int. 50:69–73. DOI: 10.1016/j.neuint.2006.06.012. PMID: 16956698. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33845307204&origin=inward.
28. Reynolds JL, Mahajan SD, Aalinkeel R, Nair B, Sykes DE, Schwartz SA. 2009; Proteomic analyses of the effects of drugs of abuse on monocyte-derived mature dendritic cells. Immunol Invest. 38:526–550. DOI: 10.1080/08820130902874110. PMID: 19811410. PMCID: PMC2812871. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=70350580814&origin=inward.
29. Longworth M, Banister SD, Mack JBC, Glass M, Connor M, Kassiou M. 2016; The 2-alkyl-2H-indazole regioisomers of synthetic cannabinoids AB-CHMINACA, AB-FUBINACA, AB-PINACA, and 5F-AB-PINACA are possible manufacturing impurities with cannabimimetic activities. Forensic Toxicol. 34:286–303. DOI: 10.1007/s11419-016-0316-y. PMID: 27547266. PMCID: PMC4971050. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84964462208&origin=inward.
30. Zhang X, Feng ZJ, Chergui K. 2015; Induction of cannabinoid- and N-methyl-D-aspartate receptor-mediated long-term depression in the nucleus accumbens and dorsolateral striatum is region and age dependent. Int J Neuropsychophar-macol. 18:pyu052. DOI: 10.1093/ijnp/pyu052. PMID: 25618403. PMCID: PMC4360221. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84939143482&origin=inward.
31. Luethi D, Kaeser PJ, Brandt SD, Krähenbühl S, Hoener MC, Liechti ME. 2018; Pharmacological profile of methylphenidate-based designer drugs. Neuropharmacology. 134(Pt A):133–140. DOI: 10.1016/j.neuropharm.2017.08.020. PMID: 28823611. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85030175626&origin=inward.
32. Caspar AT, Meyer MR, Maurer HH. 2018; Human cytochrome P450 kinetic studies on six N-2-methoxybenzyl (NBOMe)-derived new psychoactive substances using the substrate depletion approach. Toxicol Lett. 285:1–8. DOI: 10.1016/j.toxlet.2017.12.017. PMID: 29277574. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85039740351&origin=inward.
33. Tukker AM, Van Kleef RGDM, Wijnolts FMJ, De Groot A, Westerink RHS. 2020; Towards animal-free neurotoxicity screening: applicability of hiPSC-derived neuronal models for in vitro seizure liability assessment. ALTEX. 37:121–135. DOI: 10.14573/altex.1907121. PMID: 31686111. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85078387567&origin=inward.
34. Zwartsen A, Hondebrink L, de Lange DW, Westerink RHS. 2020; Hyperthermia exacerbates the acute effects of psychoactive substances on neuronal activity measured using microelectrode arrays (MEAs) in rat primary cortical cultures in vitro. Toxicol Appl Pharmacol. 397:115015. DOI: 10.1016/j.taap.2020.115015. PMID: 32320794. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85084368996&origin=inward.
35. Baskar MK, Murthy PB. 2018; Acute in vitro neurotoxicity of some pyrethroids using microelectrode arrays. Toxicol In Vitro. 47:165–177. DOI: 10.1016/j.tiv.2017.11.010. PMID: 29207333. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85036506013&origin=inward.
36. Odawara A, Saitoh Y, Alhebshi AH, Gotoh M, Suzuki I. 2014; Long-term electrophysiological activity and pharmacological response of a human induced pluripotent stem cell-derived neuron and astrocyte co-culture. Biochem Biophys Res Commun. 443:1176–1181. DOI: 10.1016/j.bbrc.2013.12.142. PMID: 24406164. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84893772545&origin=inward.
37. Hondebrink L, Kasteel EEJ, Tukker AM, Wijnolts FMJ, Verboven AHA, Westerink RHS. 2017; Neuropharmacological characterization of the new psychoactive substance metho-xetamine. Neuropharmacology. 123:1–9. DOI: 10.1016/j.neuropharm.2017.04.035. PMID: 28454981. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85019605466&origin=inward.
38. Odawara A, Matsuda N, Ishibashi Y, Yokoi R, Suzuki I. 2018; Toxicological evaluation of convulsant and anticonvulsant drugs in human induced pluripotent stem cell-derived cortical neuronal networks using an MEA system. Sci Rep. 8:10416. DOI: 10.1038/s41598-018-28835-7. PMID: 29991696. PMCID: PMC6039442. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85049841001&origin=inward.
39. Lu C, Shi X, Allen A, Baez-Nieto D, Nikish A, Sanjana NE, Pan JQ. 2019; Overexpression of NEUROG2 and NEUROG1 in human embryonic stem cells produces a network of excitatory and inhibitory neurons. FASEB J. 33:5287–5299. DOI: 10.1096/fj.201801110RR. PMID: 30698461. PMCID: PMC6436650. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85064107656&origin=inward.
40. Bright S. 2013. New and emerging drugs. Australian Drug Foundation;Melbourne: PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0035872456&origin=inward.
41. Peacock A, Bruno R, Gisev N, Degenhardt L, Hall W, Sedefov R, White J, Thomas KV, Farrell M, Griffiths P. 2019; New psychoactive substances: challenges for drug surveillance, control, and public health responses. Lancet. 394:1668–1684. DOI: 10.1016/S0140-6736(19)32231-7. PMID: 31668410. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85073696629&origin=inward.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download