Abstract
Extranodal natural killer (NK)/T-cell lymphoma, nasal type (ENKTCL-NT) is the most common subtype of Epstein-Barr virus-associated NK/T-cell lymphomas. ENKTCL-NT occurs infrequently in the gastrointestinal tract. In particular, reports of ENKTCL-on NT arising from the stomach are extremely rare. Several clusters of differentiation (CDs) have been useful in recognizing NK-cells, T-cells, and tumor cells of NK/T-cell lymphomas. Among them, the CD56 antigen is considered the most sensitive marker for ENKTCL-NT and is expressed in almost all cases of ENKTCL-NT. Thus, the development of CD56-negative ENKTCL-NT is highly atypical. This paper reports a case of a young Asian female who presented with gastric ulcer bleeding. The patient was histologically diagnosed with ENKTCL-NT. No tumor cells for CD56 were observed, whereas no monoclonality of the T-cell receptor gamma gene rearrangement was detected in the tumor cells. The patient was scheduled for systemic chemotherapy six times and achieved complete remission. Peripheral blood-hematopoietic stem cell transplantation was performed later.
Extranodal natural killer (NK)/T-cell lymphoma, nasal type (ENKTCL-NT) is an extremely rare independent disease entity classified as a subtype of non-Hodgkin’s lymphomas that originate from either NK-cells or gamma–delta (γδ) T cells and has a poor prognosis.1-3 ENKTCL-NTs are more frequently localized in the upper aerodigestive tract, such as the nasal cavity or nasopharynx, and less frequently within the nonnasal areas (the skin, salivary gland, testis, and gastrointestinal tract).4-6 Although this tumor most commonly involves the colon in the gastrointestinal tract and portions of the small intestine, gastric involvement is seldom observed.7-9 The cluster of differentiation (CD) 56 antigen is typically detected following most immunohistochemistry of tumor cells.1,4 CD56 is the most sensitive marker for ENKTCL-NT. On the other hand, there are no reports of CD56-negative ENKTCL-NT in the stomach in Korea. This paper reports a unique case of CD56-negative ENKTCL-NT, presenting as gastric ulcer bleeding.
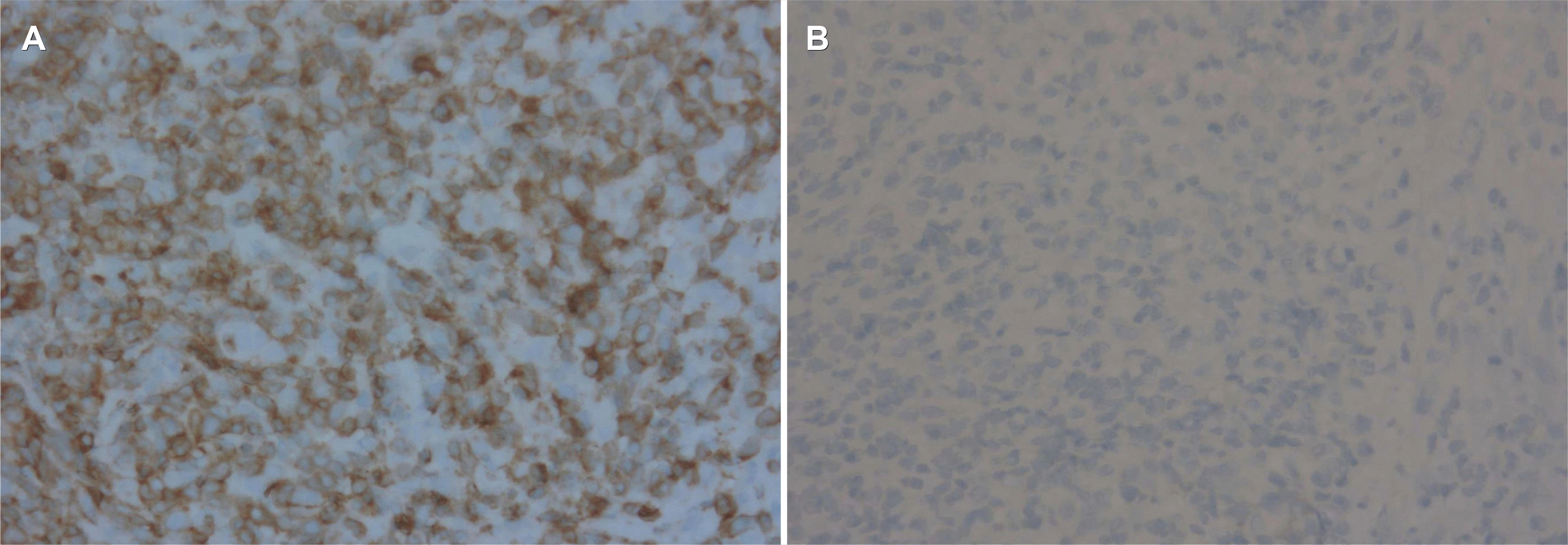
A 25-year-old woman presented with hematemesis. There was no specific prior medical history, including a malignancy. She complained of intermittent abdominal pain and discomfort for 15 days before she came to the emergency room. There were no B symptoms, such as fever, night sweats, and unintentional weight loss. Upon presentation, her blood pressure, pulse rate, and body temperature were 100/60 mmHg, 118 beats/min, and 36℃, respectively. In the physical examination, her abdomen was soft and flat. There was no cervical lymphadenopathy, no hepatosplenomegaly, or palpable mass. She also had no abdominal tenderness. The chest X-ray showed no active lung lesions in both lungs. The simple abdomen showed mild ileus, and there were no definite space-occupying lesions or significant abnormal calcifications. The complete blood count revealed normocytic normochromic anemia (hemoglobin 11.4 g/dL, and hematocrit 32.9%). In the liver function tests, the total protein (6.8 g/dL), albumin (3.8 g/dL), AST (20 U/L), and ALT (10 U/L) were normal. BUN (14 mg/dL) was normal, but the creatinine level was slightly decreased (0.48 mg/dL). LDH (171 U/L) was normal. An upper endoscopy showed an approximately 3×3 cm-sized, round, cratered ulcer with blood clots at the gastric fundus (Fig. 1). Endoscopically biopsied specimens stained with H&E showed ulceration and necrosis with infiltration of atypical lymphocytes into the gland and lamina propria (Fig. 2). Immunohistochemistry of the tumor cells in paraffin-embedded formalin-fixed specimens showed positivity for CD30, polyclonal CD3 (Fig. 3A), and T-cell-restricted intracellular antigen-1 (TIA-1) but not for monoclonal antibodies against CD20 and CD56 (Fig. 3B). In situ hybridization (EBERs-ISH) showed that some tumor cells were positive for the Epstein-Barr virus (EBV)-encoded small nuclear early RNAs (Fig. 4). PCR could not detect monoclonal T-cell receptor (TCR) γ gene rearrangement in the neoplasm.
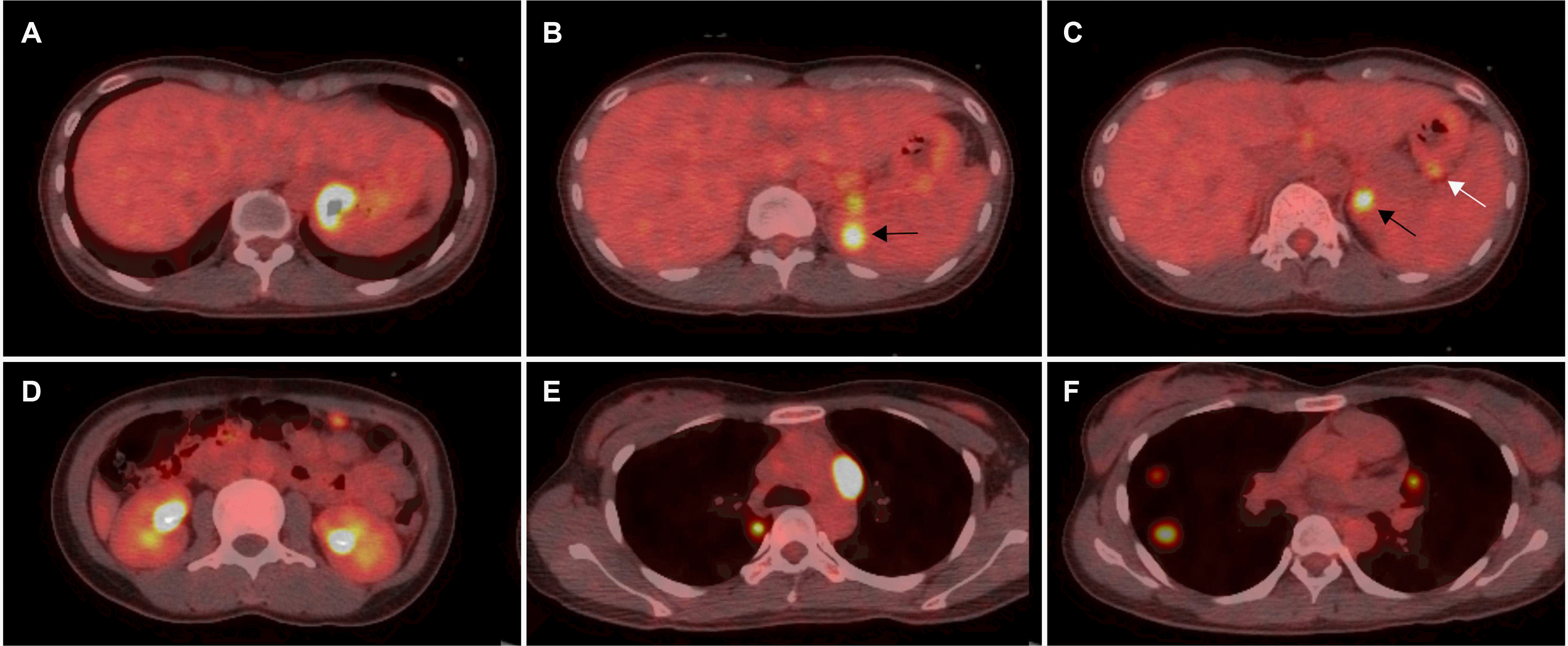
A diagnosis of ENKTCL-NT was established following pathological analysis. After referral to an oncologist, subsequent assessments were performed. PET-CT revealed the involvement of the gastric fundus, spleen, left adrenal gland, omentum of the left upper abdomen, both paratracheal lymph nodes, and the parenchyma of both lungs (Fig. 5). No bone marrow involvement was observed, and her lymphoma was classified as stage IV. After six chemotherapy cycles with nonanthracycline-containing VIDL (etoposide, ifosfamide, dexamethasone, and L-asparaginase) regimens, she achieved clinically complete remission and subsequently underwent autologous peripheral blood-hematopoietic stem cell transplantation.
While ENKTCL-NT most commonly involves the localized nasal areas, such as the upper aerodigestive tract, they may disseminate, with nasal involvement on PET-CT, to nonnasal areas, such as the skin and gastrointestinal tract.5,6 When ENKTCL-NTs primarily develop outside the nasal areas, they often present as advanced diseases rather than localized diseases without nasal involvement on PET-CT.5-7 Considering that ENKTCL-NT involving the gastrointestinal tract progresses more aggressively, particularly when the case is a primary disease, detailed studies and data have been relatively scarce compared to those for the nasal areas.5-7 A multicenter retrospective study of Asian countries published in 2013 found that among 81 patients with ENKTCL-NT involving the gastrointestinal tract, the tumor predominantly involved the small and large intestines (72%) rather than the stomach (5%).7 The small intestine was the most commonly involved site, with 42% of the cases presenting with only small intestinal disease and 28% involving both the small and large intestines.7
Based on endoscopic findings, primary gastrointestinal lymphoma, composed mainly of B-cell lymphomas, has been characterized as the ulcerative type in the upper gastrointestinal tract and polyp type in the lower gastrointestinal tract.10 An endoscopic study of NK/T-cell lymphoma among those with primary gastrointestinal lymphoma reported that the ulcer type was the most common finding, regardless of the site of involvement.11 No detailed study on the gastrointestinal endoscopic findings for ENKTCL-NT, the most common among the various subtypes of primary gastrointestinal NK/T-cell lymphoma, has been reported.
Histopathologically, the tumor showed atypical lymphocyte infiltration with an angiocentric or angiodestructive growth pattern, causing vascular occlusion, resulting in severe inflammation, such as granuloma or tissue necrosis due to ischemic damage.4-6 Therefore, the current case reveals the clinical features of the tumor, such as ulcer bleeding and perforation, depending on the organ involved.4-7 Repeated biopsy or surgical resection was required for diagnosis, considering the low tumor yield due to extensive necrosis.5,6
Oncologically, ENKTCL-NT can be classified as an EBV+ NK/T-cell lymphoma according to the etiology.2,8,12 The presence of episomal EBV genome separate from the host genome in tumor cells and EBV-latent membrane protein found in tumor cells proves that a latent EBV infection occurred early during clonal lymphomagenesis. Hence, EBV early RNAs identified via ERERs-ISH in tumor cells are essential for diagnosis, with the absence of the EBV excluding the diagnosis.1,5,6 On the other hand, given that the circulating plasma EBV DNA levels suggest apoptosis, they can be useful not as a diagnostic criterion but for a prognostic evaluation of disease progression, treatment response, recurrence, and survival rate.5,6
The current case was characterized as a gastric ulcer with bleeding on upper gastrointestinal endoscopy without the nasal presentation, EBERs-ISH+, CD56-, TIA-1+, polyclonal CD3+, and finally, no monoclonal TCR γ gene rearrangement. The TCR gene rearrangement test in the present case could have been a false-negative due to the extensive sample of necrotic tissue.13 Additionally, the T-cell lineage of our lymphoma cannot be ruled out considering the negative findings for CD56, an NK-cell marker, and positive findings for CD3, which is mainly expressed by T cells.5,6
To the best of the author’s knowledge, only two cases of upper gastrointestinal CD56-negative ENKTCL-NT have been reported thus far.14,15 Kim et al.14 reported a case of recurrence in the small intestine, particularly the duodenum, which was not a primary lesion like our case. Thus far, only Zhang et al.15 reported a primary gastric presentation, but they were not concerned with the clonal TCR gene and tumor cell lineage, unlike the present report. Moreover, considering available information regarding the typical immunophenotype of the tumor (i.e., CD56+)4-6 and the reported rates of more than 70%,4 the CD56−T-cell lineage in the present case is theoretically unique. In a study of 67 cases with ENKTCL-NT of the upper aerodigestive tract in Thailand, the putative cell origin did not affect the clinical characteristics and the treatment response. T-cell-derived tumors showed better trends in survival rates than NK-cell-derived tumors.16 No studies have reported the clinical outcomes according to tumor cell origin in the gastrointestinal tract.
In conclusion, this report described a rare case of extranodal NK/T-cell lymphoma in the stomach of a young woman who presented with hematemesis. Further results regarding the endoscopic findings, clinical features, treatment, and prognosis will emerge with continual research on ENKCL-NT.
ACKNOWLEDGEMENTS
We are deeply grateful to Prof. Jung Sik Choi, to Prof. Youn Jae Lee and to Prof. Sang Yong Seol for their valuable comments on this manuscript. Furthermore, we wish to thank Dr. Hee Won Baek, Dr. Myeong Seob Lee, Dr. Jae Han Kim, Dr. Kyutae Kim and Dr. Jae Hyuk Heo for their technical assistance.
REFERENCES
1. Nagata H, Konno A, Kimura N, et al. 2001; Characterization of novel natural killer (NK)-cell and gammadelta T-cell lines established from primary lesions of nasal T/NK-cell lymphomas associated with the Epstein-Barr virus. Blood. 97:708–713. DOI: 10.1182/blood.V97.3.708. PMID: 11157488.
2. Swerdlow SH, Campo E, Pileri SA, et al. 2016; The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 127:2375–2390. DOI: 10.1182/blood-2016-01-643569. PMID: 26980727. PMCID: PMC4874220.
3. Harabuchi Y, Takahara M, Kishibe K, Nagato T, Kumai T. 2019; Extranodal natural killer/T-cell lymphoma, nasal type: basic science and clinical progress. Front Pediatr. 7:141. DOI: 10.3389/fped.2019.00141. PMID: 31041299. PMCID: PMC6476925.
4. Vainder C, Ho J, Swerdlow SH, Akilov OE. 2016; CD56-extranodal natural killer (NK)/T-cell lymphoma, nasal type presenting as skin ulcers in a white man. JAAD Case Rep. 2:390–396. DOI: 10.1016/j.jdcr.2016.05.020. PMID: 27752533. PMCID: PMC5061065.
5. Tse E, Kwong YL. 2017; The diagnosis and management of NK/T-cell lymphomas. J Hematol Oncol. 10:85. DOI: 10.1186/s13045-017-0452-9. PMID: 28410601. PMCID: PMC5391564.
6. Tse E, Kwong YL. 2013; How I treat NK/T-cell lymphomas. Blood. 121:4997–5005. DOI: 10.1182/blood-2013-01-453233. PMID: 23652805.
7. Kim SJ, Jung HA, Chuang SS, et al. 2013; Extranodal natural killer/T-cell lymphoma involving the gastrointestinal tract: analysis of clinical features and outcomes from the Asia Lymphoma Study Group. J Hematol Oncol. 6:86. DOI: 10.1186/1756-8722-6-86. PMID: 24238138. PMCID: PMC4225665.
8. Kim EK, Jang M, Yang WI, Yoon SO. 2021; Primary gastrointestinal T/NK cell lymphoma. Cancers (Basel). 13:2679. DOI: 10.3390/cancers13112679. PMID: 34072328. PMCID: PMC8199162.
9. Li H, Lyu W. 2020; Intestinal NK/T cell lymphoma: a case report. World J Gastroenterol. 26:3989–3997. DOI: 10.3748/wjg.v26.i27.3989. PMID: 32774072. PMCID: PMC7385560.
10. Chiu KW, Changchien CS, Chuah SK, Chen CL. 1995; Endoscopic and image features in primary gastro-intestinal lymphoma: a 7-year experience. Hepatogastroenterology. 42:367–370.
11. Kim JH, Lee JH, Lee J, et al. 2007; Primary NK-/T-cell lymphoma of the gastrointestinal tract: clinical characteristics and endoscopic findings. Endoscopy. 39:156–160. DOI: 10.1055/s-2006-945114. PMID: 17657701.
12. Polyatskin IL, Artemyeva AS, Krivolapov YA. 2019; Revised WHO classification of tumors of hematopoietic and lymphoid tissues, 2017 (4th edition):lymphoid tumors. Arkh Patol. 81:59–65. DOI: 10.17116/patol20198103159. PMID: 31317932.
13. Morice WG. 2007; The immunophenotypic attributes of NK cells and NK-cell lineage lymphoproliferative disorders. Am J Clin Pathol. 127:881–886. DOI: 10.1309/Q49CRJ030L22MHLF. PMID: 17509985.
14. Kim KJ, Lee GH, Hong SS, et al. 2005; A case of duodenal relapse of Epstein-Barr virus-positive, CD56-negative extranodal NK/T-cell lymphoma, nasal type. Korean J Gastrointest Endosc. 30:204–209.
15. Zhang L, Zhang P, Wen J, Chen X, Zhang H. 2016; Primary gastric natural killer/T-cell lymphoma with diffuse CD30 expression and without CD56 expression: a case report. Oncol Lett. 11:969–972. DOI: 10.3892/ol.2015.4015. PMID: 26893677. PMCID: PMC4734030.
16. Pongpruttipan T, Sukpanichnant S, Assanasen T, et al. 2012; Extranodal NK/T-cell lymphoma, nasal type, includes cases of natural killer cell and αβ, γδ, and αβ/γδ T-cell origin: a comprehensive clinicopathologic and phenotypic study. Am J Surg Pathol. 36:481–499. DOI: 10.1097/PAS.0b013e31824433d8. PMID: 22314189.
Fig. 1
Upper gastrointestinal endoscopic examination showing a 3×3 cm-sized, single, round, cratered ulcer with blood clots at the gastric fundus.
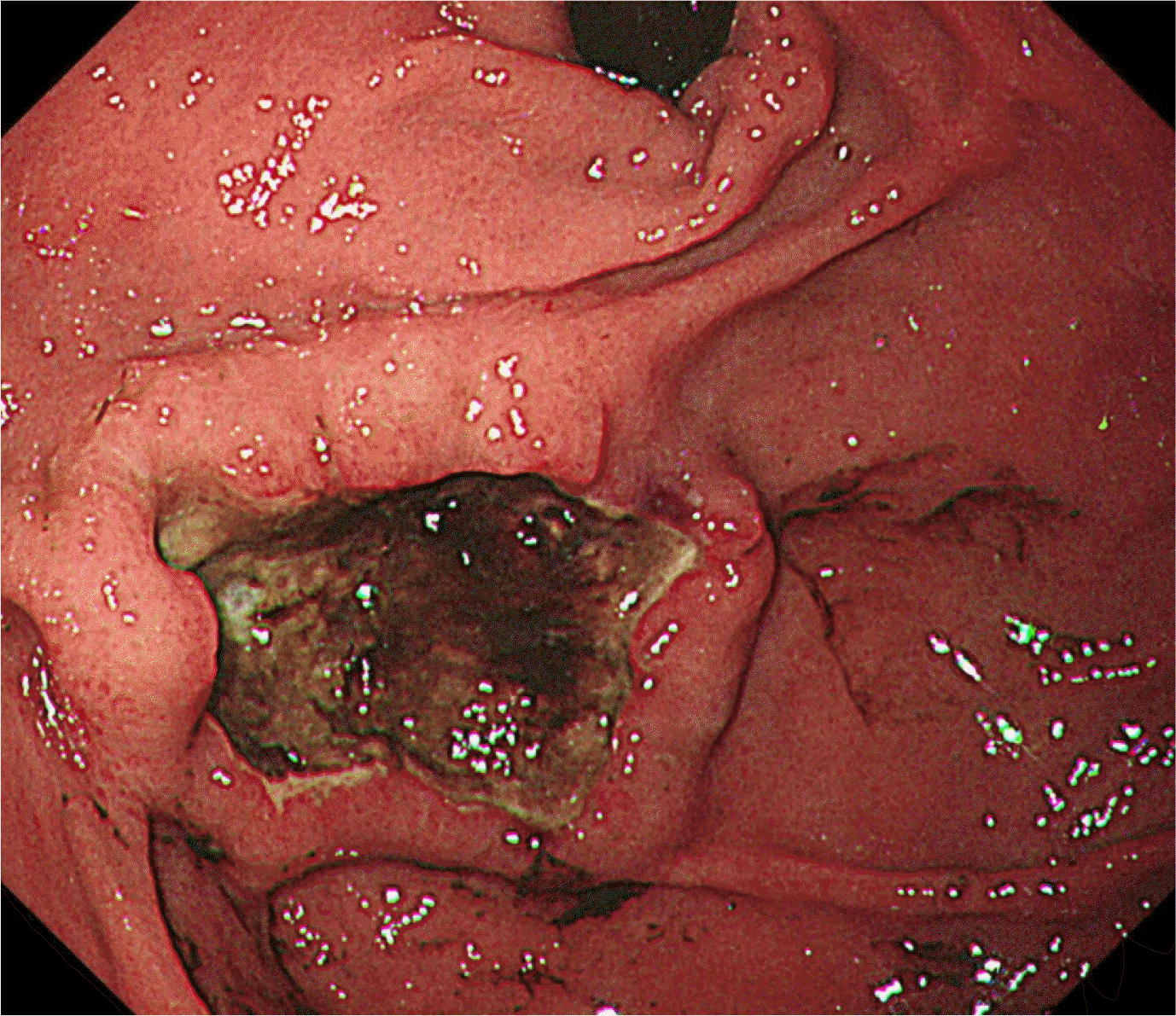
Fig. 2
Specimens stained with hematoxylin and eosin stain (H&E). (A) Specimen showing ulcer and necrosis with the infiltration of atypical lymphocytes (×100). (B) Specimen showing atypical lymphocyte infiltration into the lamina propria layer (×200).
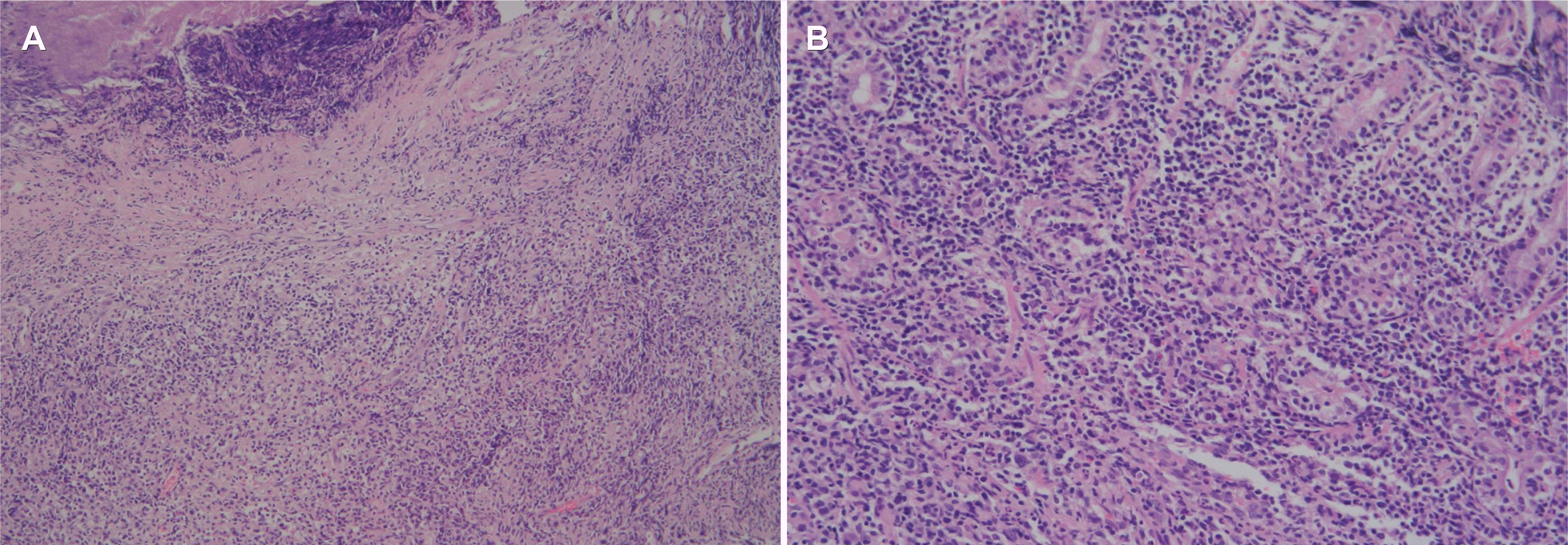
Fig. 3
Immunohistochemical staining results for tumor cells. (A) Tumor cells showing positive findings for polyclonal rabbit antihuman CD3 antibody against the CD3 antigen (×400). (B) Tumor cells showing completely negative findings for the monoclonal anti-CD56 antibody against CD56 antigen (×400).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download