Abstract
Langerhans cell histiocytosis (LCH) is reported less frequently in adults than in children. The most common site of involvement in adults is the bone, accounting for 30-50% of cases. The gastrointestinal tract is very rare, accounting for approximately 2%. We present a case of Langerhans cell histiocytosis that simultaneously invaded multiple organs, including the stomach and colon, in an adult. A 37-year-old woman with no underlying disease complained of chest discomfort and a palpable right submandibular mass. A right Level II neck mass and mediastinal LN enlargement were confirmed on the pharynx and chest CT scan. Multiple subepithelial masses with central ulceration and erosion were observed in the corpus and fundus on the esophagogastroduodenoscopy and in the right colon on the colonoscopy. The histopathology findings were the same in each tissue biopsied from the stomach, colon, and right neck lymph nodes. Langerhans cells with classical reniform nuclei and prominent eosinophils invaded the normal glands, and S100 and CD1a were positive in the immunohistochemical stain. Gastrointestinal involvement of LCH in adults is rare, asymptomatic,and can involve multiple digestive organs simultaneously, so upper endoscopy and colonoscopy should be considered for a diagnosis.
Langerhans cell histiocytosis (LCH) is a disease caused by the malignant proliferation of Langerhans cells. LCH may occur as a single lesion or may involve multiple organs. The skin and bone are the most common sites of disease in LCH. In the skin, LCH appears most commonly as a scaling rash, most frequently found on the scalp, skin folds, and the midline of the trunk. Lytic lesions can be observed in the bones, especially the skull. LCH in adults differs in the distribution of organ involvement depending on the studies.
Although LCH can occur at any age, it has been reported commonly in children, particularly one to three years of age. The incidence is estimated to be as high as five cases per million children, but it is less common in adults.1,2 Adult LCH patients with gastrointestinal involvement differ from children in the most common organs and gastrointestinal symptoms. Involvement of the duodenum and colon are common in children, and anemia and bloody stools are the most common symptoms. On the other hand, adult patients predominantly involve the colon, and half of the adult cases are asymptomatic.3
Although LCH involvement in the gastrointestinal tract is very rare (<2%), cases of LCH in the gastrointestinal tract, including the stomach, small intestine, large intestine, and peri-anus, have been reported. The endoscopic findings of LCH may differ according to the involvement site of the gastrointestinal tract. Gastric LCHs manifested in the form of ulcers or polyps, and colonic LCHs usually manifested as a single polyp without the involvement of other organs.4 There was no case of simultaneous involvement of the stomach and colon.
This paper presents a case of an adult LCH patient with simultaneous involvement of the stomach and colon, including the right cervical lymph node and mediastinal lymph node.
A 37-year-old-woman found a mass in her right submandibular region and reported chest discomfort.
She had chest pain radiating to her back 1 day before admission. She had no gastrointestinal symptoms, such as abdominal pain, epigastric soreness, nausea or vomiting, and no bleeding symptoms, such as hematemesis, melena, or hematochezia.
She had consumed alcohol approximately once a month for 20 years and smoked 0.5 packs daily.
Her white blood cell count was 9,890/mm3 (reference, 4,000-10,000/mm3), and the proportion of eosinophils was 7.0% (reference, 1-5%). The absolute eosinophil count was 692.3/mm3. The LDH level was 170 U/L (reference, 135-225 U/L). The BUN was 8.3 mg/dL (reference, 6-20 mg/dL). The serum creatine level was 0.72 mg/dL (reference, 0.5-0.9 mg/dL). The total bilirubin was 0.51 mg/dL (reference, 0-1.2 mg/dL). AST was 17 U/L (reference, 0-37 U/L). ALT was 20 U/L (reference, 0-41 U/L). The uric acid level was 4.4 mg/dL (reference, 2.4-5.7 mg/dL). There were no abnormal laboratory findings. Tumor marker tests were not performed.
The neck mass at the right level II on the pharyngeal CT was confirmed to be a 3 cm-sized lymph node (Fig. 1A, B), and an ultrasound-guided needle biopsy was performed at this lesion. In the chest enhanced CT, small to enlarged conglomerate lymph nodes in the right upper paratracheal and prevascular area (Fig. 1C, D).
Esophagogastroduodenoscopy revealed a 1.2 cm-sized protruding mass with a central depressive ulcer surrounding a raised margin with a smooth surface in the body of the greater curvature (Fig. 2A). Multiple small subepithelial tumors with central erosions were observed on the fundus (Fig. 2B). On colonoscopy, an 8 mm-sized subepithelial mass with central depression was observed in the ascending colon (Fig. 2C). A 1 cm-sized subepithelial mass with central umbilicus was observed in the distal transverse colon (Fig. 2D). Endoscopic biopsies were performed at each lesion. The PET image revealed focal FDG uptake in both hepatic lobes, both lungs, thymus, and right ovary, including the multiple lymph nodes of the right cervical level II and VI, upper paratracheal, right external iliac, and right inguinal areas and right upper thigh intermuscular area. A bone marrow biopsy and bone scan were performed because of the abnormal findings in the pelvic bone and bilateral proximal femurs on the whole body MRI, but there was no clear evidence of LCH invasion in the bone marrow biopsy and bone scan.
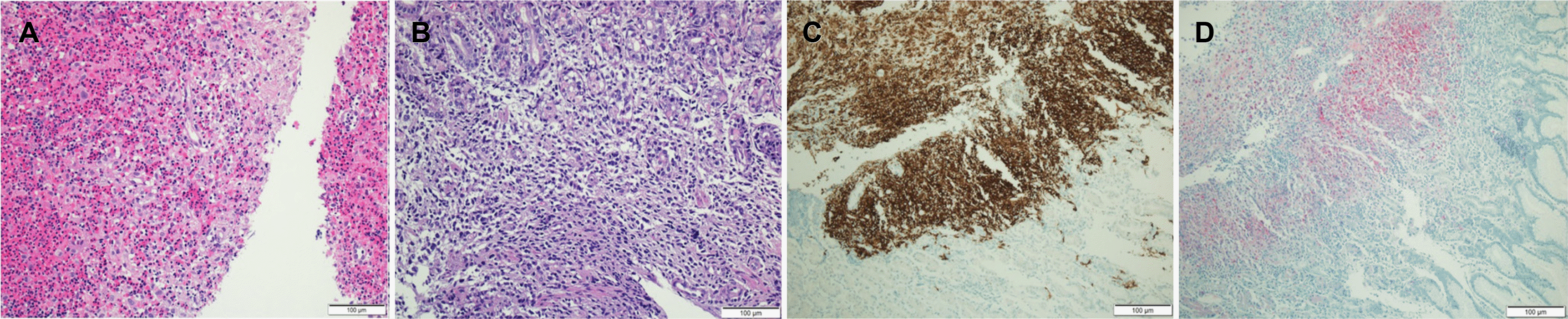
Tissues biopsied from the stomach, colon, and neck lymph nodes showed the same pathological findings. Focal aggregation of histiocytic cells with abundant eosinophils was observed in the lamina propria and muscularis mucosa, myofascial lamina propria, and the myofascial mucosa (Fig. 3A, B). Langerhans cells were verified in each tissue sample, and CD1a and S-100 protein were positive in the immunochemical stain. Similarly, samples obtained from gastroscopy and colonoscopy stained positive for S-100 protein and CD1a (Fig. 3C, D). Cytomegalovirus, acid-fast bacillus stain, PCR, and the culture for tuberculosis performed by biopsy were all negative.
The final diagnosis was Langerhans cell histiocytosis that invaded the stomach and colon, including both hepatic lobes, both lungs, thymus, right ovary, and multiple lymph nodes of the right cervical level II and VI, upper paratracheal, right external iliac and right inguinal areas and right upper thigh intermuscular area.
LCH can invade multiple organs. Therefore, she started induction chemotherapy with cytarabine and vincristine. After the 7th cycle of chemotherapy, CT for follow-up showed a decrease in the size of the liver and multiple lymph nodes, but the lung lesions progressed. She is currently taking oral mercaptopurine and methotrexate as an outpatient. A follow-up CT scan 12 months after diagnosis revealed improvement in all lesions, including the lungs, but complete remission was not reached.
Langerhans cell histiocytosis (LCH) is characterized by the infiltration of one or more organs by large mononuclear cells. In LCH, Langerhans cells can be mixed with various inflammatory cells, such as eosinophils, polynuclear cells, plasma cells, lymphocytes, and mononuclear cells. The cytoplasm of Langerhans cells is abundant and eosinophilic. In staining for the S100 protein, Langerhans cells show strong positivity in the cell membrane and cell nucleus, and CD1a staining is also expressed relatively well in Langerhans cells. Birbeck granules (tennis racket inclusions) can be seen on electron microscopy.5
LCH is common in children, and the most common site is the bone, accounting for 30-50%. On the other hand, gastrointestinal invasion is rare, accounting for only 2% of cases.6 According to the international histiocyte society registry,2 which included 274 adult LCH patients from 13 countries, the gender ratio was similar (143:126), and the mean age at diagnosis was 33 years (SD 15 years) for men and 35 years (SD 14 years) for women. Among all adult LCH patients, 31.4% (86/274) had a single-system disease, and 44 patients had isolated pulmonary involvement of LCH. On the other hand, there were 68.6% (188/274) of LCH cases with multiple organ involvement. Although previous cases of LCH involved a single organ involvement in the gastrointestinal tract,7 the present case was an adult LCH patient with simultaneous involvement of the stomach and colon, including the lymph nodes in the right neck and mediastinum. Despite the presence of ulcerative subepithelial lesions in the stomach and colon, there were no gastrointestinal symptoms in this case. In previous studies, approximately half of adult LCH patients with GI infiltration were asymptomatic.3 Although adult LCH is usually asymptomatic, pediatric LCH often presents with gastrointestinal symptoms, such as loose stools, abdominal pain, and vomiting.7
LCH is diagnosed using the histological criteria established by the Histological Society in 1987. There are no pathognomonic symptoms or radiographic features of LCH; hence, pathologic confirmation is required for diagnosis.6 On histological examination, abundant eosinophils are observed together with histocytes.1 An immunohistochemical test with positive S-100 and CD1a can demonstrate LCH.5 LCH can involve any organ or system in the body. Therefore, biopsies of all suspected sites are required for a diagnosis.8 Endoscopy may provide an opportunity for tissue collection and confirmation of gastrointestinal involvement because gastrointestinal invasion is possible even in patients without gastrointestinal symptoms.
Treatment is based on the severity and extent of LCH. The treatment of adult LCH follows the treatment of children. Although the regimen for LCH is unclear in adults, vinblastine/ prednisone according to the standard pediatric protocol was similar in efficacy and toxicity in adults.9 Patients with low-risk LCH have a good prognosis and a high long-term survival rate of 99%. In one study, followed by a median of 28 months after diagnosis, 15 patients (6.4%) had died (death rate, 1.5/100 person-years; 95% CI, 0.9-2.4). The probability of survival at 5 years postdiagnosis was 92.3% (95% CI, 85.6-95.9) overall, 100% for patients with single-system disease (n=37), 87.8% (95% CI, 54.9-97.2) for isolated pulmonary disease (n=34), and 91.7% (95% CI, 83.6-95.9) for multisystem disease (n=163). Survival was similar among patients with multisystem disease, with or without liver or lung involvement; the 5-year survival was 93.6% (95% CI, 84.7-97.4) versus 87.5% (95% CI, 65.5-95.9), respectively; p-value=0.1).2 In contrast, the survival rates for high-risk LCH patients were close to 80%. Typically, adult patients present with limited skin or bone involvement that can be treated with surgical excision or local radiation therapy, resulting in an overall survival rate of 100%.10
Endoscopy should be considered in adult LCH patients without gastrointestinal symptoms because multiple gastrointestinal involvement, including the stomach and colon, is possible without symptoms.
REFERENCES
1. Rao DG, Trivedi MV, Havale R, Shrutha SP. 2017; A rare and unusual case report of Langerhans cell histiocytosis. J Oral Maxillofac Pathol. 21:140–144. DOI: 10.4103/jomfp.JOMFP_10_17. PMID: 28479703. PMCID: PMC5406796.
2. Aricò M, Girschikofsky M, Généreau T, et al. 2003; Langerhans cell histiocytosis in adults. Report from the International Registry of the Histiocyte Society. Eur J Cancer. 39:2341–2348. DOI: 10.1016/S0959-8049(03)00672-5.
3. Singhi AD, Montgomery EA. 2011; Gastrointestinal tract langerhans cell histiocytosis: a clinicopathologic study of 12 patients. Am J Surg Pathol. 35:305–310. DOI: 10.1097/PAS.0b013e31820654e4. PMID: 21263252.
4. Shankar U, Prasad M, Chaurasia OP. 2012; A rare case of langerhans cell histiocytosis of the gastrointestinal tract. World J Gastroenterol. 18:1410–1413. DOI: 10.3748/wjg.v18.i12.1410. PMID: 22493557. PMCID: PMC3319970.
5. Gulati N, Allen CE. 2021; Langerhans cell histiocytosis: version 2021. Hematol Oncol. 39 Suppl 1(Suppl 1):15–23. DOI: 10.1002/hon.2857. PMID: 34105821. PMCID: PMC9150752.
6. Jezierska M, Stefanowicz J, Romanowicz G, Kosiak W, Lange M. 2018; Langerhans cell histiocytosis in children - a disease with many faces. Recent advances in pathogenesis, diagnostic examinations and treatment. Postepy Dermatol Alergol. 35:6–17. DOI: 10.5114/pdia.2017.67095. PMID: 29599667. PMCID: PMC5872238.
7. Lee SJ, Hwang CS, Huh GY, Lee CH, Park DY. 2015; Gastric Langerhans cell histiocytosis: case report and review of the literature. J Pathol Transl Med. 49:421–423. DOI: 10.4132/jptm.2015.05.19. PMID: 26056155. PMCID: PMC4579285.
8. Haupt R, Minkov M, Astigarraga I, et al. 2013; Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 60:175–184. DOI: 10.1002/pbc.24367. PMID: 23109216. PMCID: PMC4557042.
9. Allen CE, Ladisch S, McClain KL. 2015; How I treat Langerhans cell histiocytosis. Blood. 126:26–35. DOI: 10.1182/blood-2014-12-569301. PMID: 25827831. PMCID: PMC4492195.
10. Grana N. 2014; Langerhans cell histiocytosis. Cancer Control. 21:328–334. DOI: 10.1177/107327481402100409. PMID: 25310214.
Fig. 1
Computed tomography (CT) findings of the right neck lymph node and mediastinal lymph node (black triangular arrows). (A) 3 cm-sized lymph node at the right level II neck on the axial view of the pharyngeal CT. Axial view. (B) Coronal view of the pharyngeal CT. (C) Conglomerated multiple small to enlarged lymph nodes in right upper paratracheal and prevascular area on the axial view of the chest CT. (D) Coronal view of the chest CT.
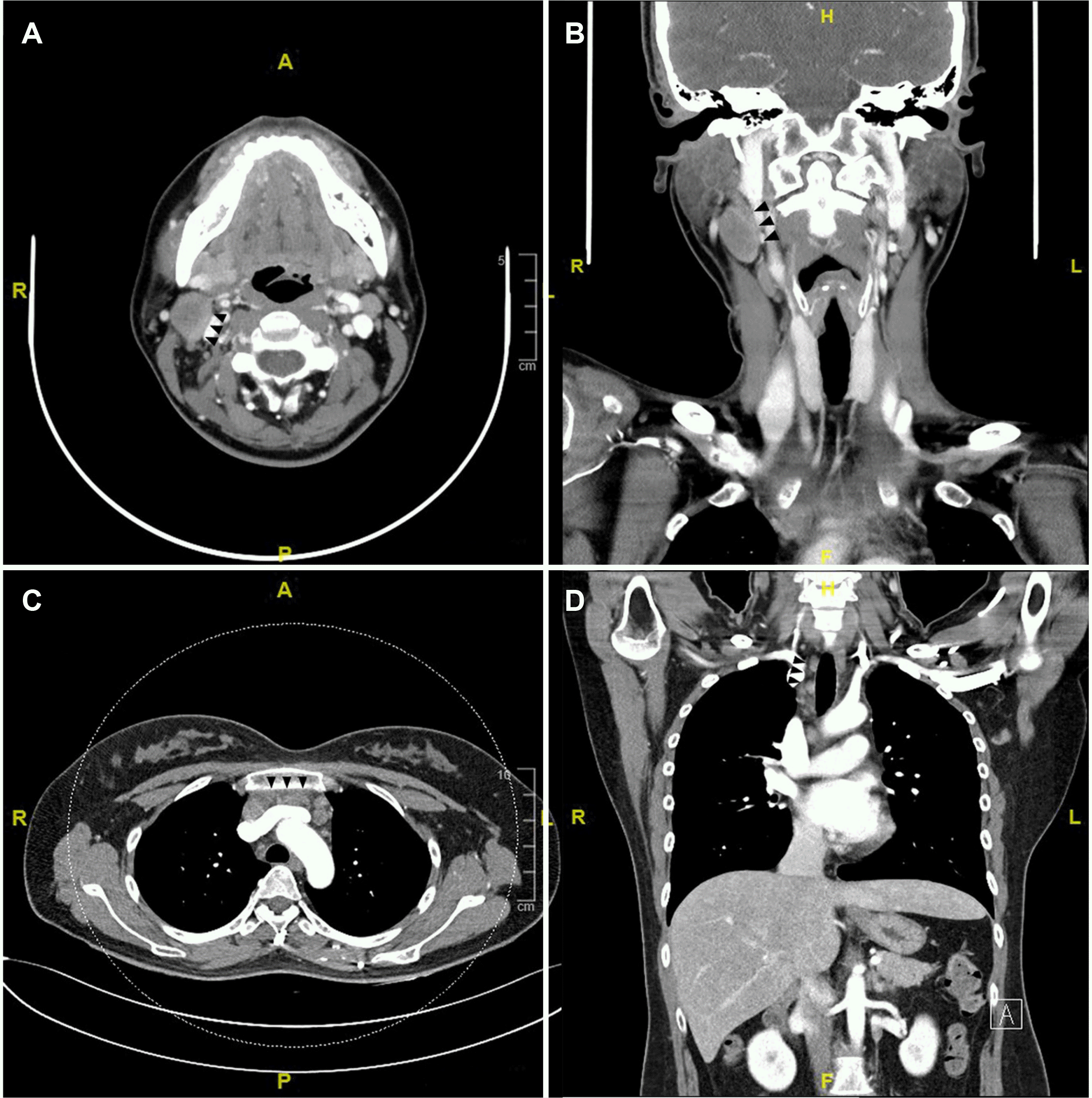
Fig. 2
Endoscopic findings of multiple involvement in the stomach and colon in adult Langerhans cell histiocytosis patients. (A) Multiple subepithelial tumors with central erosion on fundus. (B) 1.2 cm sized ulcerative mass with elevated margin on the great curvature of the body. (C) Subepithelial tumor with central hyperemic erosion on the ascending colon. (D) Subepithelial tumor with central umbilicus on the transverse colon.
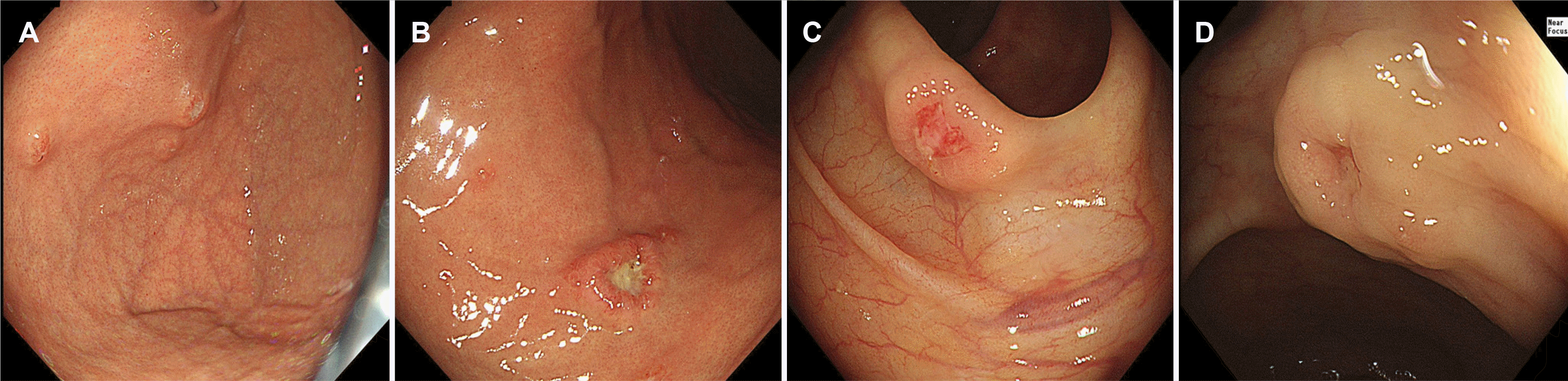
Fig. 3
Pathology findings of the right neck lymph node and gastric ulcerative lesion. (A) Pathologic findings of the right neck lymph node (hematoxylin and eosin stain [H&E], ×100). (B) Pathologic findings of the gastric ulcerative lesion (H&E, ×100). (C) CD1a staining was positive (×100). (D) S-100 staining was positive (×100).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download