INTRODUCTION
Colonoscopy is an important diagnostic and therapeutic tool for various colonic diseases because it allows visualization of the mucosa of the entire large intestine.
1,2 Effective bowel cleansing is essential before performing a colonoscopy to ensure accurate diagnosis and treatment.
3,4 Poor bowel preparation can lead to longer procedure times that could make the patients uncomfortable and cause missed lesions; this is a major cause of interval cancer.
5-7 Patient compliance with bowel cleansing agents and efficacy are correlated with the adequacy of bowel preparation.
8 Therefore, efforts have been made to develop bowel cleansing agents with a low volume for patient compliance. Currently, diverse bowel cleansing agents, such as polyethylene glycol (PEG) and sodium phosphate (NaP), oral sulfate solution (OSS), and sodium picosulfate with magnesium citrate (SPMC) are used in preparation for colonoscopy.
3,9-13
However, low volume agents, such as NaP, OSS, and SPMC, can cause dehydration and electrolyte imbalance, such as hyponatremia in elderly patients,
14,15 and are reported to have worse preparation quality than the PEG.
15-17 In contrast, the PEG solution has become the preferred bowel cleansing agent owing to its safety and efficacy. On the other hand, its large volume and poor taste can cause several adverse effects, such as abdominal fullness, nausea, vomiting, and abdominal pain, which can reduce patient compliance.
11,15,16 A PEG-ascorbic acid (PEG-Asc) formulation was developed to reduce the volume of PEG that needs to be ingested and improve the taste. In this PEG-Asc formulation, ascorbic acid was added to the existing PEG formulation.
18 Currently, the split dose of 2-L PEG-Asc is the most widely used standard bowel cleansing method worldwide.
19,20
Recently, several 1-L PEG-Asc variations have been developed, which have a lower volume (total 2-L including free water 1-L) than conventional 2-L PEG-Asc (total 3-L including free water 1-L) and are more tolerable for the patient. This leads to improved patient compliance. Indeed, several studies assessed the efficacy and safety of 1-L PEG-Asc compared to conventional 2-L PEG-Asc, showing a favorable colon cleansing efficacy with comparable safety.
21-23 Therefore, the use of 1-L PEG-Asc is expanding, and several 1-L PEG-Asc agents have been introduced with diverse compositions of PEG3350 contents, electrolytes, and ascorbate (
Supplementary Table 1). On the other hand, few studies have investigated the efficacy, safety, and compliance of 1-L PEG-Asc agents and their satisfaction with them, particularly in clinical practice.
This study compared the efficacy, safety, and patient satisfaction of two types of 1-L PEG-Asc (CleanViewAL® [Tae Joon Pharmaceutical Company, Seoul, Korea] and Plenvu® [Norgine, Harefield, United Kingdom]) as bowel cleansing agents in healthy adults.
DISCUSSION
Effective bowel cleansing for colonoscopy is associated with an accurate diagnosis, safe inspection, and appropriate treatments, e.g., in removing precancerous lesions.
3,7 Many factors affect the quality of the colonoscopy besides bowel preparation agents, such as patients’ age, degree of physical activity, adherence to instructions, underlying disease, continuing medical education of the endoscopist, instrument quality, and withdrawal times.
6,27,28 On the other hand, among the several factors mentioned, the bowel cleansing agent is considered the most important factor, and the discomfort experienced in the bowel preparation process affects patient compliance. Therefore, studies on bowel cleansing agents are continuously being conducted. Although the PEG solution has an excellent cleansing effect and safety evaluation, its large volume makes patients uncomfortable. 1-L PEG-Asc products are being developed to reduce the volume of bowel cleansing agents. Plenvu
® is the first 1-L PEG-Asc that has reduced the PEG3350 content and increased the ascorbate content (instead of the 2-L PEG-Asc). Phase 3 studies have compared the safety and effectiveness of bowel cleansing between Plenvu
® and other bowel preparation agents.
29-31 In the MORA study, which compared Plenvu
® with the split-dose method of 2-L PEG-Asc, Plenvu
® showed superior colon cleansing efficacy with comparable safety and tolerability.
21,30 In other studies, Plenvu
® was as effective as OSS or SPMC in achieving overall bowel cleansing success. Only the adverse event rates were slightly higher with Plenvu
® than with OSS, and Plenvu
® showed superior high-quality cleansing of the right colon compared with SPMC.
29-31 Right colon preparation is essential because detection of adenoma and serrated polyps is more difficult in the right colon than in other areas of the colon.
27 Moreover, multicenter, observational phase 4 studies also confirmed that 1-L PEG-Asc (Plenvu
®) could be a reasonable substitute for 2-L PEG-Asc.
23,32 In previous studies, no serious side effects were found with Plenvu
®, but because a small volume causes diarrhea, hypernatremia may occur when dehydrated because of high doses of sodium ascorbate and sodium sulfate.
31 CleanViewAL
® is 1-L PEG-Asc with a reduced sodium content compared to Plenvu
®. In the present study, the efficacy and safety of 1-L PEG-Asc products were evaluated, and comparisons were made between CleanViewAL
® (1-L PEG-Asc) and Plenvu
® (1-L PEG-Asc). The total BBPS scores of both groups were similar and remarkably high (8.67±1.00 in the CleanViewAL
® group and 8.70±0.76 in the Plenvu
® group [p=0.869]), indicating that both 1-L PEG-Asc agents are very effective in terms of bowel cleansing. In addition, the CleanViewAL
® group is not inferior to the Plenvu
® group in aspects of bowel cleansing. In the present study, aged patients and patients with underlying diseases, such as IBD or renal dysfunction, were excluded owing to the lack of experience with 1-L PEG-Asc agents in the Seoul Paik Hospital. On the other hand, recent studies reported the effectiveness and safety of 1-L PEG-Asc agents in diverse settings, such as in patients with IBD33 and even in elderly patients and patients with renal dysfunction.
34
Both groups showed sufficient ADR (over 40%). The ADR was calculated in patients with screening colonoscopy, and 35.9% and 39.1% were observed in the CleanViewAL® group and Plenvu® group, respectively. Only patients under 60 years were enrolled in this study due to safety concerns in aged patients, which resulted in an ADR lower than 40%. On the other hand, an ADR of approximately 40% in this study population is reasonable considering the average age. In all, both 1-L PEG-Asc agents can be used to achieve successful bowel cleansing. The overall satisfaction was also similar in the two groups; however, the CleanViewAL® group had a significantly higher “taste” satisfaction score than Plenvu® (p=0.028). The increased ascorbic acid in CleanViewAL® might make it taste better than Plenvu®.
Regarding safety, no cases of severe adverse events that required hospitalization were observed in either group. In terms of laboratory test result changes, the Plenvu
® group showed significant elevation in sodium levels after bowel cleansing, whereas CleanViewAL
® showed no difference in sodium levels before and after bowel cleansing. This may be due to differences in the sodium contents between the two products (
Supplementary Table 1). In addition, sodium, chloride, creatinine, eGFR, and liver function test (AST, ALT) also showed significant differences before and after bowel cleansing in both groups, but there were no cases of critical changes exceeding CTCAE grade I. In addition, there was no clinical symptom related to these laboratory changes. Therefore, these two agents are safe for bowel cleansing in average-aged patients. In the present study, CleanViewAL
® was not inferior to Plenvu
® in terms of bowel cleaning and tolerability in adults without chronic disease, suggesting that CleanViewAL
®, like Plenvu
®, can be an alternative to 2-L PEG-Asc in average-aged patients.
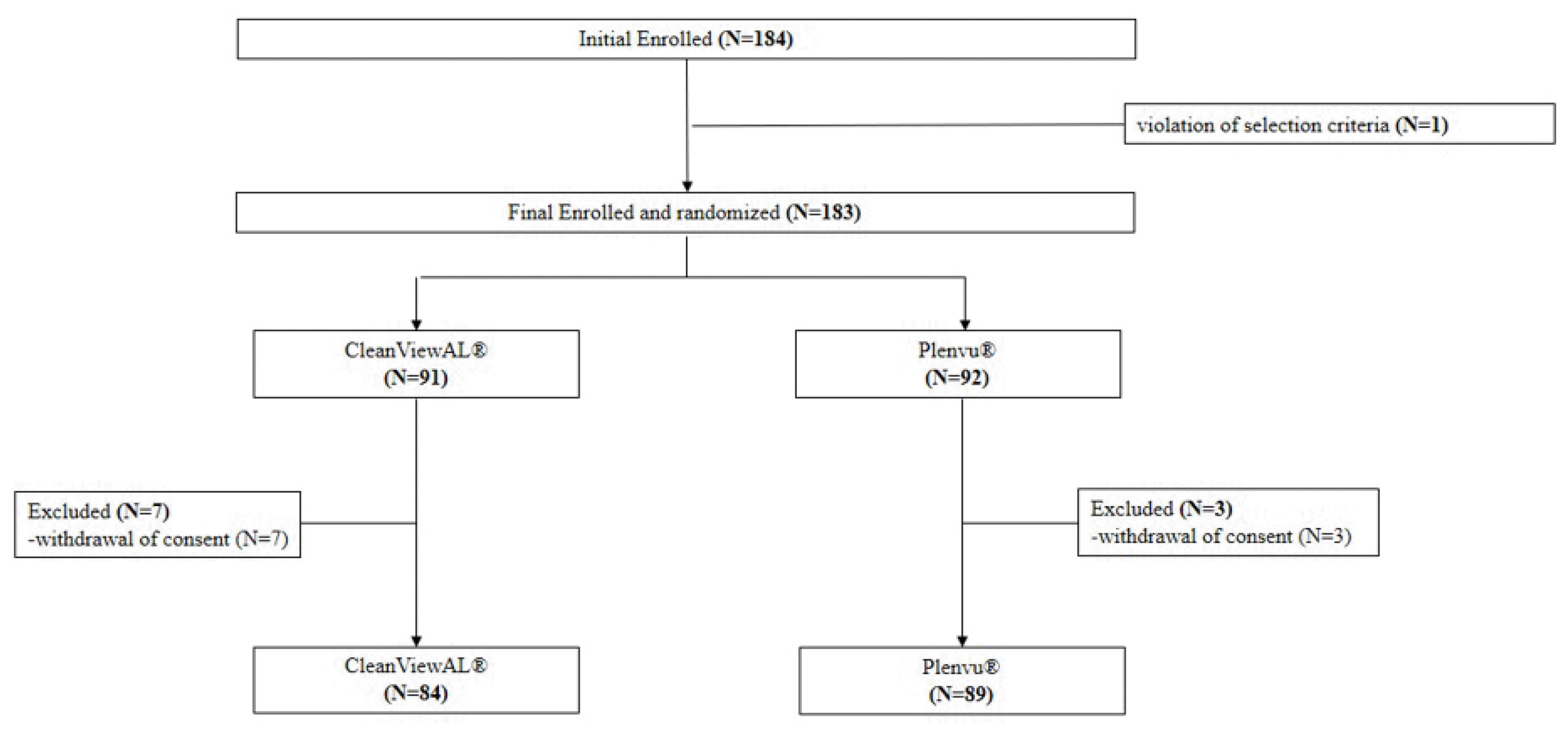
This study had some limitations. First, the dropout rate was predicted to be 5%; however, the COVID-19 pandemic affected the patient dropout rate. Several patients canceled their colonoscopy appointment during the COVID-19 pandemic period. Therefore, the final dropout rate was increased to 5.46% (10/183); the CleanViewAL
® group (7.69%, 7/91) had a higher dropout rate than the Plenvu
® group (3.26%, 3/92), despite the patients being assigned randomly to the two groups. However, the efficacy demonstrated by the BBPS score was very similar in both groups, suggesting that CleanViewAL
® group is not inferior to that of the Plenvu
® group. Second, this study was conducted in an open-label, single-center manner. It is surmised that the open-label method can affect the patient’s satisfaction. Nevertheless, patients with no experience of 1-L PEG-Asc were included in this study. Therefore, previous experience may affect the “volume” satisfaction score but not that of “taste”. Third, this study only included average-aged patients. Although a few studies reported the efficacy and safety of 1-L PEG-Asc for aged patients or patients with chronic disorders,
33,34 the authors have limited experience on 1-L PEG-Asc for aged patients or patients with chronic disorders. With the safety concerns, aged patients and patients with chronic disorders were excluded from this study. Therefore, using 1-L PEG-Asc for aged patients or patients with chronic disorders cannot be guaranteed.
In conclusion, compared to the standard 2-L PEG-Asc regimen, both 1-L PEG-Asc agents have a much smaller volume for the patient to consume and less volume to make the patient uncomfortable. These findings suggest that both types of 1-L PEG-Asc, CleanViewAL® and Plenvu®, are effective and safe bowel cleansing agents in average-aged adults. CleanViewAL® was preferred in terms of “taste” satisfaction.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download