Abstract
Ischemic colitis (IC) is an underreported chronic disease characterized by the hypoperfusion of the bowel mucosa. The diagnosis and treatment may be challenging because its clinical course resembles other colitis or even colorectal malignancies. This paper reports an untypical case to underline the diversity of IC manifestation. A 68-year-old man with several comorbidities was admitted because of abdominal pain with a 6-month duration and a mass in the left lower quadrant. Colonoscopy revealed erosive pseudomembranous colitis narrowed colon segments with ulcerated mucosa mimicking colorectal cancer and inflammatory bowel disease. The stool cultures and Clostridium difficile toxin tests were negative. After the failure of conservative therapy, the Hartmann procedure with temporary ileostomy was performed uneventfully. The histological results of the surgical specimens revealed IC with focal pseudomembranous areas.
Ischemic colitis (IC) is one of the most frequent forms of ischemic bowel disease, with various presentations depending on the injury's onset, duration, and extent of involvement in colon segments.1-3 A submucosal mass and a narrowed colon segment with an ulcerated mucosa have been reported to mimic colorectal cancer and inflammatory bowel disease during the clinical and endoscopic examination.4-6 In addition to being caused mostly by a severe Clostridium difficile infection (CDI), pseudomembranous colitis (PMC) is characterized by yellow–white nodules or plaques and can be a consequence of other disease processes, including severe IC.7,8 This paper presents an untypical IC case to highlight its various manifestations and raise awareness of this condition among the other infectious colitis in tropical countries.
A 68-year-old man was referred to the Gia-Dinh People’s Hospital for a 6-month history of left lower quadrant abdominal pain associated with diarrhea, intermittent bloody mucus per rectum, and fecal incontinence. The pain began gradually and was moderate in severity. His loss of appetite and 9.98 kg in weight in a couple of months were also reported. No fever or night sweating was noticed. His comorbidities included type 2 diabetes, hypertension, ischemic stroke, benign prostatic hyperplasia, and psoriasis. Consequently, he was prescribed Gliclazide, Telmisartan, Amlodipine, Atorvastatin, and Fexofenadine. Neither IBD nor colorectal cancer was documented in his family history. At the primary facility, he had been empirically prescribed metronidazole (2 weeks), ciprofloxacin (2 weeks), albendazole (2 days), and ivermectin (2 days). Unfortunately, the symptoms worsened, so he was admitted to the Gia-Dinh People’s Hospital. The physical examination revealed a distended abdomen with tenderness in the left lower quadrant and a palpable 10×12 cm mass without guarding or rebound tenderness. The standard blood tests showed leukocytosis (WBC 13,520/μL), a CRP level of 99.2 mg/L, a hemoglobin level of 14.6 g/dL, and normal kidney-liver function tests. Cytomegalovirus-IgM/IgG, antinuclear antibody, rheumatoid factor, and anti-dsDNA tests were negative. C. difficile A and B toxin PCR-based detection assays returned negative. The stool was examined, and a microbiological evaluation returned negative for ova, parasites, or other bacteria causative agents. Fecal calprotectin was 126 μg/g. Abdominal CT detected a local abnormal colonic wall thickness up to 15 mm focally from the rectum to the splenic flexure. In addition, there were neither punctate nor serpiginous calcifications of the mesenteric veins nor increased density in the fatty tissue of the surrounding mesentery on the CT-scan imaging. The combs sign was positive and with serosal infiltration (Fig. 1A). A later CT angiography with 3D reconstruction showed diffuse atherosclerosis of multivessel without any acute intestinal ischemia signs (Fig. 1B). Flexible sigmoidoscopy revealed the large-bowel hypomotility, severe pseudomembranous proctosigmoiditis, and ulcero-infiltrative mass-like lesion resulting in severely inflamed non-traversable stricture at the splenic flexure (Fig. 2A-C). The histopathology from biopsies obtained at multiple colon segments displayed unspecific moderate chronic active inflammation, mild hyalinization of the lamina propria, with microthrombi, without granulomas, cytopathic viral inclusion, and dysplasia (Fig. 3A). Nevertheless, the pseudomembrane may not be discovered owing to a lack of adequate biopsies. Because of the pseudomembrane in the endoscopic results and the past antibiotic usage, for a presumptive C. difficile infection, an empirical course of oral vancomycin, mucosal healing agents, and parenteral feeding was commenced. On the other hand, the symptoms (bloody mucus diarrhea, fecal incontinence) and the mass resolved insufficiently. On a physical examination, he was severely wasted with no abdominal rebound tenderness. Consequently, a repeat colonoscopy showed that the colonic mucosa had been healing completely at the rectum. Nevertheless, the other segment had friable, ischemic, and necrotic-like appearances (Fig. 2D-F). Previously observed pseudomembranes were not visualized. His plasma albumin level decreased from 31 to 17 g/dL, reflecting malnutrition and severe protein loss enteropathy. Owing to the nonresolution of his symptoms, a successful laparoscopic Hartmann procedure was performed with a temporary ileostomy. Grossly, an examination of the resection segment showed the thickened wall of the sigmoid colon with strictures, ulcerations, and pseudomembranes. A microscopic examination revealed extensive mucosal, and submucosal hyalinization with ulceration, and chronic inflammation consistent with chronic IC. Neither calcification in the vascular nor suggestive signs of myointimal hyperplasia was confirmed by H&E staining (Fig. 3B, C). The patient had recovered well postoperatively and tolerated the diet orally with gradual advancement. After 2 months, he had a colonoscopy revealing no synchronous colorectal neoplasms. Four months after his original operation, he had an ileostomy reversal procedure. He recovered well and eventually gained 4.54 kg.
According to the Declaration of Helsinki by the World Medical Association, the study is under the Gia-Dinh People’s Hospital and with ethical and legal principles (according to the Declaration of Helsinki by the World Medical Association). The patient has given his written informed consent to publish his case.
Although IC is one of the most prevalent types of ischemic bowel disease, it may be underrated in tropical areas where infectious colitis predominates. This is the first IC case reported in the Vietnamese population to the authors’ knowledge. A diagnosis of IC is based on multi-aspects (history, physical examination, risk factors, imaging modalities, endoscopic and pathologic evidence). IC had diverse manifestations, ranging from acute colitis (including PMC) to chronic colitis or complications (stricture, gangrenous bowel, and fulminant pancolitis.2,3,9 Like previous case reports, the patient, who was elderly with various comorbidities related to atherosclerosis, such as hypertension, ischemic strokes, and diabetes, had affected colon segments on the left side.2,3,9
Colonoscopy has been regarded as the gold standard diagnostic approach by directly observing the colonic mucosa and obtaining biopsy specimens, which can overcome the difficulty of recognizing IC.2,3 Common features include darkened or pale, edematous and fragile mucosa, segmental erythema, petechial hemorrhages, longitudinal ulcer, and lesions that are particularly segmented and distributed patchily.1-3 The endoscopic examination revealed nonspecific pseudomembranous colitis with ulcers from the rectum to the splenic flexure. PMC is distinguished by raised yellow-white nodules or plaque on the mucosal surfaces of the colon that form pseudomembranes.7,8 In the case of IC, despite being uncommon, PMC is not a novel phenomenon and may be induced by hypoperfusion.7,8,10,11 Early ischemia induces punctuate pseudomembrane development. Nevertheless, confluent pseudomembranes can be observed as the injury advances. Acute inflammatory cells, nuclear debris, fibrin, and other inflammatory elements from lamina propria make up these pseudomembranes.7,10
Empiric antibiotics therapy and parenteral feeding were commenced owing to the strong association of PMC with CDI, the risk of false-negative C. difficile tests due to the previous empiric antibiotics treatment, and the extensive history of the patient’s symptoms. His symptoms persisted, even with escalating treatment. Therefore, a repeated colonoscopy showed that the pseudomembrane had resolved incompletely, and the mucosa appeared paler and more friable than non-C. difficile causes of pseudomembranous colitis had been investigated. Those characteristics were more in line with IC. The microscopic features from colonic specimens were collected during the colonoscopy and surgery to differentiate IC from CDI-associated colitis and other types of colitis.11,12 The degree of heterogeneity seen on the histological examination was associated with the severity of ischemic damage and the time since the initial injury.12 In particular, in PMC, the presence of a hyalinized lamina propria was both a sensitive and specific diagnostic for IC. At the same time, crypt atrophy might not be typical.12 An ischemic origin was also suggested by lamina propria hemorrhage, full-thickness mucosal necrosis, and layering of pseudomembranes in a limited colon distribution.12 In this situation, initially, colonoscopy, the depth of the mucosal sample may have been inadequate due to the avoidance of colon perforation when obtaining a biopsy in the friable mucosa region. Accordingly, the initial biopsies were inconsistent with the typical histology features to exclude CDI or infectious colitis completely. On the other hand, both hyalinizations of the lamina propria and atrophic crypts on the final surgical specimens were observed. As a result, there was a probability that the biopsy specimens were insufficient for the histologic investigation, which might have prevented determining the precise entity at the initial admission. On the other hand, infectious colitis, particularly by parasitic etiologies, was the most common one in our tropical setting, so infectious causes after diagnosing IC should be ruled out.
Besides, a mass-like appearance similar to a colon tumor may be associated with severe submucosal and mural edema during a physical examination. Although the IC is initially suspected of being colon carcinoma only in a few cases, it was challenging to rule out a malignant diagnosis in our elderly patient.4,11,13 Despite the endoscopic and CT features indicating a carcinoma, the biopsy results in all cases were negative for a malignancy.4,6,11,13 Moreover, in some cases, the follow- up colonoscopies performed several weeks later showed the resolution of those masses.4 In the present case, he had undergone a screening colonoscopy after ostomy reversal surgery without colorectal neoplasm synchronously.
Regarding the imaging modality, a CT scan can be helpful in a diagnosis of IC. Among the frequently reported features, such as colonic wall thickening, edema, bowel dilatation, target sign, and effusion of intestinal circumference, his abdomen CT scan revealed a positive combs sign, a well-known sign in Crohn's disease imaging.2,3 This sign refers to multiple- tubular, tortuous opacities on the mesenteric side of the ileum aligned as the teeth of a comb on contrast-enhanced CT.14 His comb sign may indicate an active, progressive, and extensive inflammatory condition in the clinical, histological, and endoscopic aspects. Mesenteric angiography was not performed in this case because most cases of IC are transient and non-occlusive.2,3,9 Furthermore, for IC, a careful differential diagnosis is crucial, mainly to differentiate it from phlebosclerotic colitis, a rare fatal condition, or rare disorders, such as idiopathic myointimal hyperplasia of the mesenteric veins. These were excluded because the CT-scan imaging revealed no punctate or serpiginous calcifications of the mesenteric veins.15 Moreover, the histology examination did not detect myointimal hyperplasia of the mesenteric veins in H&E staining, and IC is also infrequent in pseudomembrane form.16 Unfortunately, the elastin staining cannot be performed at the Gia-Dinh People’s Hospital due to resource constraints. This is the limitation of this case report. In the present case, infectious colitis, particularly CDI, might overlap and superimpose on the preceding chronic ischemia condition. This may lead to the atypical clinical course of IC in tropical areas. The management of IC varies depending on the severity of the condition.2,3,9
Most cases should be treated conservatively with close monitoring if there is no evidence of gangrene, perforation, bleeding, or peritonitis. On the other hand, early surgical consultation is indicated for patients with systemic disease and failure of conventional therapy. Moreover, an intraoperative colonoscopy can clarify whether a resection is appropriate.2,5,6 The development of hypoalbuminemia might predict the gangrenous changes in CI.2,9 Initially, the patient had moderate IC with optimized medical management. On the other hand, an early elective segmental colonic resection with primary anastomosis was indicated given the severity and deleterious evolution of the patient’s condition with significant hypoalbuminemia and gangrenous and friable colon mucosa. This procedure helps prevent peritonitis and obtain the appropriate specimens for a definitive diagnosis despite the absence of irrational peritoneal signs during the examination.
In summary, in tropical areas, the coexistence of infectious colitis can aggravate a preexisting ischemic condition. Challenging scenarios, such as this case, where the clinical characteristics of many conditions overlap, reaffirm the necessity to integrate data in a multidisciplinary setting, including the patient risk factors, compatible history, physical examination, endoscopic appearance, and well-sampled biopsy during colonoscopy and surgery. Raising physician awareness and vigilance about this disease is critical for establishing an early diagnosis and prompt management, particularly in elderly individuals presenting with abdominal pain, chronic diarrhea, rectal bleeding, and the presence of comorbid disease.
ACKNOWLEDGEMENTS
We are grateful to our colleagues from Nhan Dan Gia Dinh Hospital in Ho Chi Minh City's Department of Gastroenterology, Gastroenterology Surgery, Radiology, and Pathology for their invaluable assistance.
REFERENCES
1. Azam B, Kumar M, Mishra K, Dhibar DP. 2019; Ischemic colitis. J Emerg Med. 56:e85–e86. DOI: 10.1016/j.jemermed.2019.01.016. PMID: 30850260.
2. Hung A, Calderbank T, Samaan MA, Plumb AA, Webster G. 2019; Ischaemic colitis: practical challenges and evidence-based recommendations for management. Frontline Gastroenterol. 12:44–52. DOI: 10.1136/flgastro-2019-101204. PMID: 33489068. PMCID: PMC7802492.
3. Xu Y, Xiong L, Li Y, Jiang X, Xiong Z. 2021; Diagnostic methods and drug therapies in patients with ischemic colitis. Int J Colorectal Dis. 36:47–56. DOI: 10.1007/s00384-020-03739-z. PMID: 32936393. PMCID: PMC7493065.
4. Khor TS, Lauwers GY, Odze RD, Srivastava A. 2015; "Mass-forming" variant of ischemic colitis is a distinct entity with predilection for the proximal colon. Am J Surg Pathol. 39:1275–1281. DOI: 10.1097/PAS.0000000000000438. PMID: 26034865.
5. Killebrew SR, Patel NJ, Lam JP, Zhai Q, Lewis MD. 2020; It's not always cancer: ischemic colitis masquerading as a colonic mass. Gastrointest Endosc. 91:954–955. DOI: 10.1016/j.gie.2019.11.006. PMID: 31733198.
6. Kim HS, Sauri F, Kang JH, Kim NK. 2020; Mass-forming ischemic colitis that mimics colon cancer. Chin Med J (Engl). 133:1253–1254. DOI: 10.1097/CM9.0000000000000800. PMID: 32433062. PMCID: PMC7249714.
7. Farooq PD, Urrunaga NH, Tang DM, von Rosenvinge EC. 2015; Pseudomembranous colitis. Dis Mon. 61:181–206. DOI: 10.1016/j.disamonth.2015.01.006. PMID: 25769243. PMCID: PMC4402243.
8. Tang DM, Urrunaga NH, De Groot H, von Rosenvinge EC, Xie G, Ghazi LJ. 2014; Pseudomembranous colitis: not always caused by Clostridium difficile. Case Rep Med. 2014:812704. DOI: 10.1155/2014/812704. PMID: 25214850. PMCID: PMC4151585.
9. Brandt LJ, Feuerstadt P, Longstreth GF, Boley SJ. 2015; ACG clinical guideline: epidemiology, risk factors, patterns of presentation, diagnosis, and management of colon ischemia (CI). Am J Gastroenterol. 110:18–45. DOI: 10.1038/ajg.2014.395. PMID: 25559486.
10. Montoro MA, Brandt LJ, Santolaria S, et al. 2011; Clinical patterns and outcomes of ischaemic colitis: results of the Working Group for the Study of Ischaemic Colitis in Spain (CIE study). Scand J Gastroenterol. 46:236–246. DOI: 10.3109/00365521.2010.525794. PMID: 20961178.
11. Lorber J, Pessegueiro A. Ischemic colitis masquerading as pseudomembranous colitis. Proceedings of UCLA Healthcare. 2014; 18.
12. Dignan CR, Greenson JK. 1997; Can ischemic colitis be differentiated from C difficile colitis in biopsy specimens? Am J Surg Pathol. 21:706–710. DOI: 10.1097/00000478-199706000-00011. PMID: 9199649.
13. Danakas AM, Fazili BG, Huber AR. 2019; Mass-forming ischemic colitis: a potential mimicker of malignancy. Case Rep Pathol. 2019:8927872. DOI: 10.1155/2019/8927872. PMID: 31007961. PMCID: PMC6441522.
14. Madureira AJ. 2004; The comb sign. Radiology. 230:783–784. DOI: 10.1148/radiol.2303020645. PMID: 14990842.
15. Chen W, Zhu H, Chen H, et al. 2018; Phlebosclerotic colitis: our clinical experience of 25 patients in China. Medicine (Baltimore). 97:e12824. DOI: 10.1097/MD.0000000000012824. PMID: 30412073. PMCID: PMC6221691.
16. Song SJ, Shroff SG. 2017; Idiopathic myointimal hyperplasia of mesenteric veins of the ileum and colon in a patient with crohn's disease: a case report and brief review of the literature. Case Rep Pathol. 2017:6793031. DOI: 10.1155/2017/6793031. PMID: 28894617. PMCID: PMC5574263.
Fig. 1
Computed tomography (CT) findings. (A) Abdominal CT showed diffuse wall thickening of the splenic flexure wall-thickening, and no calcifi cations within the bowel wall and mesentery vein were observed, left colon segments with serosal infiltration (arrows). (B) 3D reconstruction from CT angiography revealed the diffuse atherosclerosis of multivessel but no severe stenosis in the main abdominal branches of the aorta.
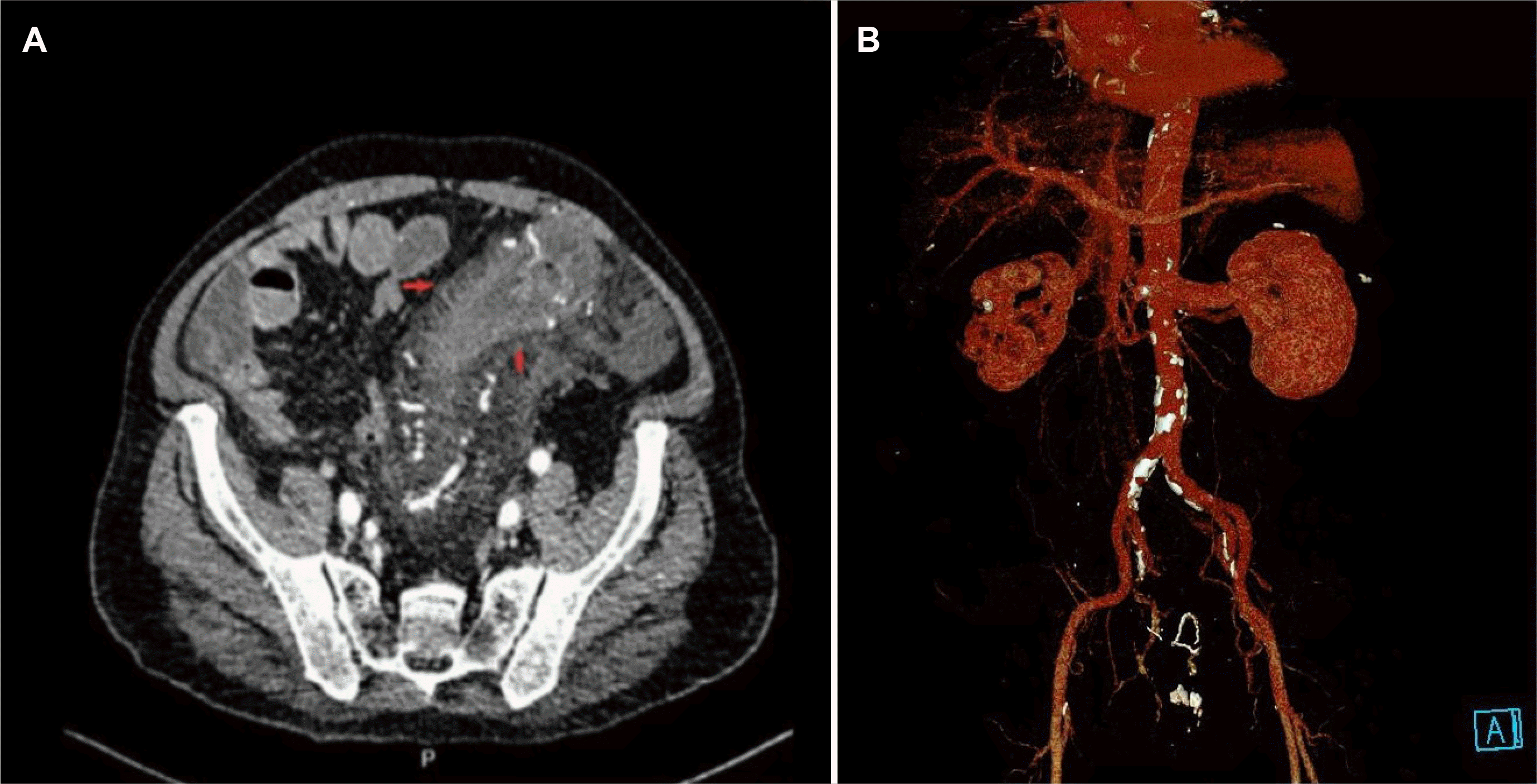
Fig. 2
Images from flexible sigmoidoscopy showing. (A-C) Before treatment, the ischemic bowel segments had dilated lumens, yellowish exudate (pseudomembranes), ulcers on the mucosal surface, and an ulcero-infiltrative mass-like lesion caused by a severely inflamed non-traversable stricture at the splenic flexure. (D-F) After treatment, the mucosa of the colon segments became friable and ulcerated with necrotic-like appearances.
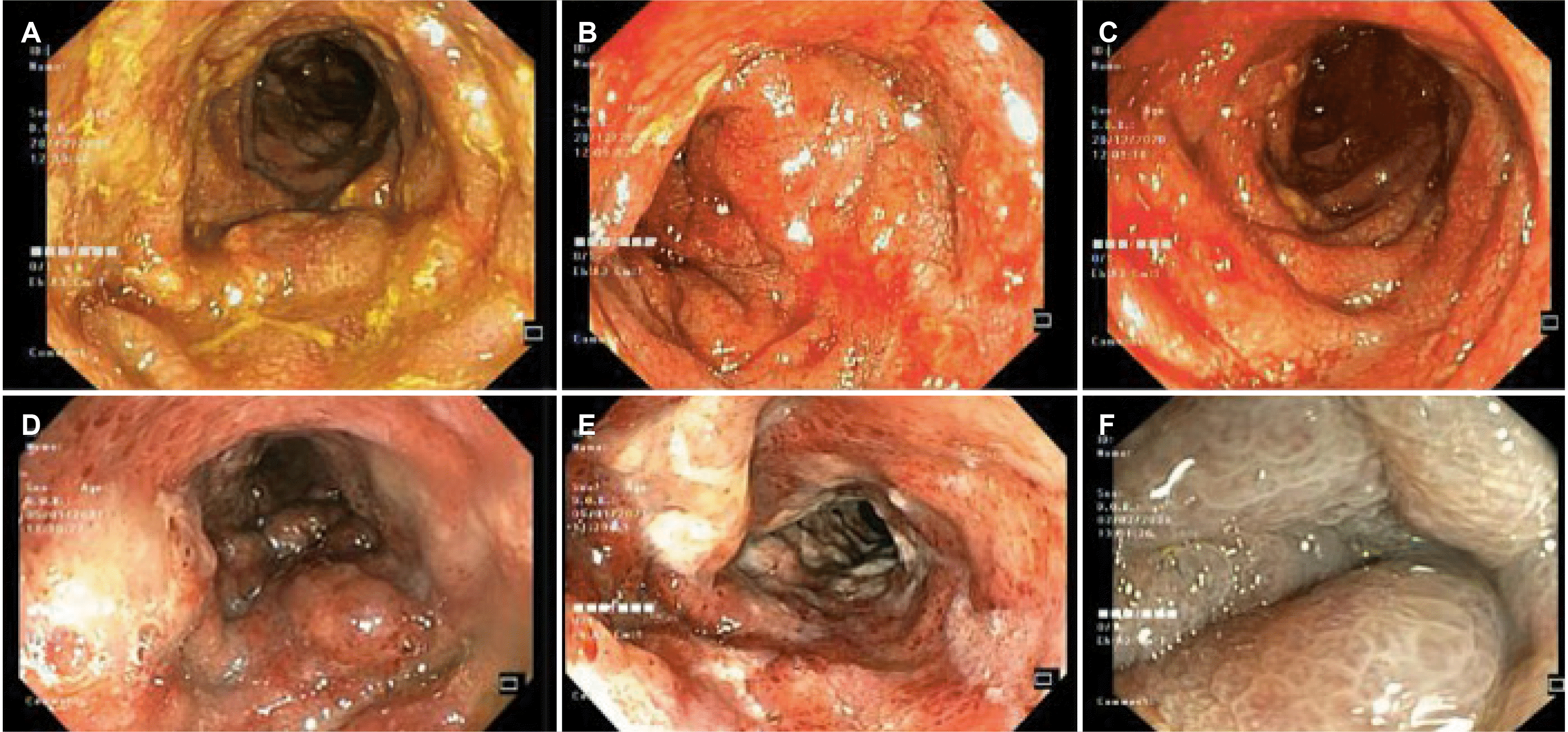
Fig. 3
Histopathology examination of the endoscopic and surgical specimens. (A) Endoscopic biopsy specimens revealed unspecific moderate chronic active inflammation, mild hyalinization of the lamina propria, with microthrombi, without granulomas, cytopathic viral inclusion, and dysplasia (hematoxylin and eosin stain [H&E], ×100). The final surgical specimens showed the following: (B) marked acute inflammatory exudate on the mucosa (pseudomembranous appearance) (white arrow and black arrows), crypt abscess and atrophy, mucosal necrosis (stars) (H&E, ×40). (C) Ischemic colitis with ischemic changes including mucosal edema, lamina propria hemorrhage (white arrows), lamina propria hyalinization & capillary dilatation (black arrows) (H&E, ×200).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download