Abstract
Background/Aims
Symptom-based subtyping of functional dyspepsia (FD) is used to segregate patients into groups with homogenous pathophysiological mechanisms. This study examined whether subtyping could reflect the duodenal and gastric microinflammation in FD patients.
Methods
Twenty-one FD patients without Helicobacter pylori infection were recruited. An endoscopic biopsy was performed in the duodenum 2nd portion, stomach antrum, and body. The eosinophil and mast cell counts per high-power field (×40) were investigated by H&E and c-kit staining, respectively. The degree of inflammatory cell infiltration, atrophy, and intestinal metaplasia was also determined by H&E staining in the stomach. The baseline characteristics and eosinophil and mast cell infiltrations were compared among the three groups (epigastric pain syndrome, postprandial distress syndrome, and overlap).
Results
According to the symptom assessment, seven subjects were classified into the epigastric pain syndrome group, 10 into the postprandial syndrome group, and four into the overlap group. The baseline variables were similar in the three groups. Eosinophil infiltration was more prominent in the duodenum than in the stomach. In contrast, mast cell infiltration was similar in the duodenum and stomach. The eosinophil counts in the duodenum were similar in the three groups. The eosinophil counts in the stomach and mast cell counts in the duodenum and stomach were also similar in the three groups.
Functional dyspepsia (FD) is a common disorder characterized by the presence of chronic or recurrent symptoms of upper abdominal pain or discomfort in the absence of known specific structural causes.1 Several pathophysiological mechanisms have been suggested to underlie dyspeptic symptoms, including impaired gastric accommodation, delayed gastric emptying, impaired mucosal permeability, low-grade inflammation, gastric hypersensitivity, Helicobacter pylori (H. pylori) infection, altered response to duodenal lipids or acid, abnormal duodenojejunal motility, and central nervous system dysfunction.2
Although targeting the underlying pathophysiological mechanisms is ideal for therapeutic strategies, tests for mechanism evaluation are usually impractical, and single predominant symptoms cannot predict disease mechanisms accurately.3-6 The Rome IV criteria suggest using symptom-based subtypes, such as postprandial stress syndrome (PDS) and epigastric pain syndrome (EPS), based on the assumption that subtyping could segregate patients with FD into homogenous mechanisms groups.7 Many practice guidelines refer to symptom-based subtypes for choosing the first-line treatment option, such as proton pump inhibitors for EPS and prokinetics for PDS.8-10
Over the last decade, emerging data point towards the duodenal microinflammation (most notably eosinophilic duodenitis and intestinal mast cell disease) as a crucial underlying pathophysiological mechanism of FD.11-14 As treatments targeting gastric sensorimotor function are of limited efficacy,15-17 duodenal pathology should be addressed from the aspect of the therapeutic target. Thus, the present study examined the differences in eosinophil and mast cell infiltration in the duodenum and stomach according to the symptom-based subtypes of FD.
This retrospective study included patients with FD who satisfied the Rome IV criteria and underwent upper endoscopy with gastric and duodenal 2nd portion biopsy for investigating H. pylori infection and duodenal inflammation. The patients were excluded if they had H. pylori infection or had undergone previous abdominal surgery. The diagnosis of a H. pylori infection was made using Giemsa staining for the endoscopic biopsy samples from the gastric antrum and body.
This study protocol was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (IRB) at Samsung Medical Center, Seoul, Korea, on the 15th of April 2013 (IRB No. 2020-12-093). The requirement for informed consent was waived because routinely collected de-identified data were used.
From the chart review, the demographic characteristics (age and sex), symptoms of FD, and presence of other diseases (gastroesophageal reflux disease, irritable bowel syndrome [IBS], hypertension, and diabetes) were obtained. Subjects were classified into three symptom-based subtypes of FD (EPS, PDS, and overlap).7 All endoscopic images were reviewed by an experienced endoscopist (Min YW), and the status of reflux esophagitis, Hill grade,18 and chronic atrophic gastritis was determined. The chronic atrophic gastritis grade was diagnosed by evaluating the atrophic border in the endoscopic images. The atrophic pattern system described by Kimura et al.19 was used.
An experienced pathologist (Ahn S), blinded to the clinical information, investigated the gastric and duodenal pathology of previously obtained unstained biopsy samples. The eosinophil and mast cell counts per high-power field (HPF, ×40) were investigated by H&E and c-kit staining, respectively. The counts were determined by the total number of eosinophils and mast cells in the five non-overlapping HPFs. The degrees of inflammatory cell infiltration, atrophy, and intestinal metaplasia were also determined by H&E staining of two biopsy sites (gastric antrum and body). Atrophy was defined as a decrease in the number of appropriate glands. Inflammatory cell infiltration and metaplastic and non-metaplastic atrophy were determined using the updated Sydney system scores (i.e., 0=none, 1=mild, 2=moderate, and 3=marked).
Statistical analyses were conducted using GraphPad Prism 9.2.0 software (GraphPad, San Diego, CA, USA). The baseline characteristics were compared among groups using one-way analysis of variance (ANOVA) with multiple comparisons and the χ2 test. The cell counts were compared among groups using the Kruskal-Wallis test and one-way ANOVA with multiple comparisons. Statistical significance was defined as a two-sided p-value <0.05.
Twenty-one subjects were included in this study. According to the symptom assessment, seven subjects were classified into the EPS group, 10 into the PDS group, and four into the overlap group (Table 1). The baseline characteristics (age, sex, presence of hypertension, diabetes, IBS, reflux esophagitis, and Hill grade) were similar in the three groups. The stomach pathology (inflammation, atrophy, and intestinal metaplasia in the antrum and body) was similar in the three groups (Table 2).
The mast cell counts were similar in the duodenum and the stomach (Table 3, Fig. 1D-F). Although the three groups showed similar mast cell counts in the duodenum (p=0.076) and stomach (antrum, p=0.245; body, p=0.058), the stomach body mast cell counts were higher in the overlap group than in the EPS and PDS groups (adjusted p=0.027 and p=0.012, respectively) (Fig. 1F).
Although FD is a common disorder worldwide, the clinical outcomes are unsatisfactory. One of the reasons might be the heterogeneous pathophysiological mechanisms in the patient group. To increase the therapeutic efficacy, physicians attempt to determine the mechanisms of the disease through symptom assessment in usual practice and perform physiological tests in selected cases. Symptom-based subtypes, such as PDS and EPS, are believed to be more homogenous in terms of symptom-generating mechanism than the entire group of FD.20 Recently, duodenal pathology, such as eosinophilic infiltration, has been suggested as an important mechanism for FD.14,21 Accordingly, it is essential to determine if the conventional symptom-based subtypes could also reflect the status of the duodenal pathology. The eosinophils and mast cell counts in the duodenum and stomach were examined according to symptom-based subtypes of FD, and no differences were found among the subtypes. If the duodenal pathology is an important symptom-generating mechanism of FD, it should be utilized in the treatment strategies. Nevertheless, these findings show that the conventional symptom-based subtypes used for an FD treatment strategy do not reflect the duodenal pathology. Although more confirmative studies are required, the symptom-based subtypes will need to be modified.
Duodenal eosinophilic infiltration may induce gastroduodenal dysmotility via eosinophil-induced T-cell activation and subsequent release of leukotrienes.22-24 Indeed, several studies have shown increased duodenal eosinophil counts in patients with FD compared to healthy controls.12,13,25 On the other hand, some studies reported no significant association between duodenal eosinophilia and FD. Therefore, the presence of duodenal eosinophilic degranulation may be more important.25,26 In an Australian study, increased duodenal mucosal eosinophil counts were not associated with FD but with postprandial symptoms (postprandial fullness and early satiety).12 By contrast, the present study found no association between the duodenal eosinophilic count and symptom-based subtypes of FD. Mast cell infiltration could be involved in the symptom-generating mechanism of FD.13,27 Duodenal mast cell infiltration may be linked to IBS.28 In the present study, an increased tendency of the mast cell count was observed in the overlap group. A possible explanation is that the overlapping group may share IBS characteristics.
This study had some limitations. This study was retrospective. Thus, the results are limited in terms of generalization. Eosinophil degranulation was not investigated. Activated eosinophils may be more important in the FD pathophysiology. Nevertheless, this study investigated the differences in eosinophil and mast cell infiltration in patients with FD according to symptom-based subtypes. In addition, the present study has a small sample size and no healthy control data. Although the differences in eosinophil and mast cell infiltration in the duodenum and stomach were examined according to symptom-based subtypes of FD, comparable data may be obtained using a control group. Subjects with a H. pylori infection by staining may be excluded to reduce confounding effects. Nevertheless, it would be better to consider the past eradication history together. The small sample size lowers the power of the results and limits detailed analysis, but the negative observations could help design future large prospective studies.
In conclusion, the duodenal eosinophil and mast cell counts were similar according to the symptom-based subtypes of FD. This suggests that the currently used subtyping of FD does not reflect the duodenal eosinophil and mast cell infiltration. Thus, a new subtyping of FD must include novel emerging pathophysiology.
Notes
REFERENCES
1. Talley NJ, Stanghellini V, Heading RC, Koch KL, Malagelada JR, Tytgat GN. 1999; Functional gastroduodenal disorders. Gut. 45 Suppl 2(Suppl 2):II37–II42. DOI: 10.1136/gut.45.2008.ii37. PMID: 10457043. PMCID: PMC1766695.
2. Tack J, Bisschops R, Sarnelli G. 2004; Pathophysiology and treatment of functional dyspepsia. Gastroenterology. 127:1239–1255. DOI: 10.1053/j.gastro.2004.05.030. PMID: 15481001.
3. Doran S, Jones KL, Andrews JM, Horowitz M. 1998; Effects of meal volume and posture on gastric emptying of solids and appetite. Am J Physiol. 275:R1712–R1718. DOI: 10.1152/ajpregu.1998.275.5.r1712. PMID: 9791094.
4. Mundt MW, Hausken T, Samsom M. 2002; Effect of intragastric barostat bag on proximal and distal gastric accommodation in response to liquid meal. Am J Physiol Gastrointest Liver Physiol. 283:G681–G686. DOI: 10.1152/ajpgi.00499.2001. PMID: 12181183.
5. Karamanolis G, Caenepeel P, Arts J, Tack J. 2006; Association of the predominant symptom with clinical characteristics and pathophysiological mechanisms in functional dyspepsia. Gastroenterology. 130:296–303. DOI: 10.1053/j.gastro.2005.10.019. PMID: 16472585.
6. Bouras EP, Delgado-Aros S, Camilleri M, et al. 2002; SPECT imaging of the stomach: comparison with barostat, and effects of sex, age, body mass index, and fundoplication. Single photon emission computed tomography. Gut. 51:781–786. DOI: 10.1136/gut.51.6.781. PMID: 12427776. PMCID: PMC1773479.
7. Stanghellini V, Chan FK, Hasler WL, et al. 2016; Gastroduodenal disorders. Gastroenterology. 150:1380–1392. DOI: 10.1053/j.gastro.2016.02.011. PMID: 27147122.
8. Enck P, Azpiroz F, Boeckxstaens G, et al. 2017; Functional dyspepsia. Nat Rev Dis Primers. 3:17081. DOI: 10.1038/nrdp.2017.81. PMID: 29099093.
9. Miwa H, Kusano M, Arisawa T, et al. 2015; Evidence-based clinical practice guidelines for functional dyspepsia. J Gastroenterol. 50:125–139. DOI: 10.1007/s00535-014-1022-3. PMID: 25586651. PMCID: PMC8831363.
10. Oh JH, Kwon JG, Jung HK, et al. 2020; Clinical practice guidelines for functional dyspepsia in Korea. J Neurogastroenterol Motil. 26:29–50. DOI: 10.5056/jnm19209. PMID: 31917913. PMCID: PMC6955183.
11. Walker MM, Salehian SS, Murray CE, et al. 2010; Implications of eosinophilia in the normal duodenal biopsy - an association with allergy and functional dyspepsia. Aliment Pharmacol Ther. 31:1229–1236. DOI: 10.1111/j.1365-2036.2010.04282.x. PMID: 20222916.
12. Walker MM, Aggarwal KR, Shim LS, et al. 2014; Duodenal eosinophilia and early satiety in functional dyspepsia: confirmation of a positive association in an Australian cohort. J Gastroenterol Hepatol. 29:474–479. DOI: 10.1111/jgh.12419. PMID: 24304041.
13. Wang X, Li X, Ge W, et al. 2015; Quantitative evaluation of duodenal eosinophils and mast cells in adult patients with functional dyspepsia. Ann Diagn Pathol. 19:50–56. DOI: 10.1016/j.anndiagpath.2015.02.001. PMID: 25735567.
14. Wauters L, Talley NJ, Walker MM, Tack J, Vanuytsel T. 2020; Novel concepts in the pathophysiology and treatment of functional dyspepsia. Gut. 69:591–600. DOI: 10.1136/gutjnl-2019-318536. PMID: 31784469.
15. Talley NJ, Tack J, Ptak T, Gupta R, Giguère M. 2008; Itopride in functional dyspepsia: results of two phase III multicentre, randomised, double-blind, placebo-controlled trials. Gut. 57:740–746. DOI: 10.1136/gut.2007.132449. PMID: 17965059.
16. Hallerbäck BI, Bommelaer G, Bredberg E, et al. 2002; Dose finding study of mosapride in functional dyspepsia: a placebo-controlled, randomized study. Aliment Pharmacol Ther. 16:959–967. DOI: 10.1046/j.1365-2036.2002.01236.x. PMID: 11966505.
17. Tack J, Van Den Elzen B, Tytgat G, et al. 2009; A placebo-controlled trial of the 5-HT1A agonist R-137696 on symptoms, visceral hypersensitivity and on impaired accommodation in functional dyspepsia. Neurogastroenterol Motil. 21:619–e24. DOI: 10.1111/j.1365-2982.2008.01260.x. PMID: 19220756.
18. Hill LD, Kozarek RA, Kraemer SJ, et al. 1996; The gastroesophageal flap valve: in vitro and in vivo observations. Gastrointest Endosc. 44:541–547. DOI: 10.1016/S0016-5107(96)70006-8. PMID: 8934159.
19. Kimura K, Satoh K, Ido K, Taniguchi Y, Takimoto T, Takemoto T. 1996; Gastritis in the Japanese stomach. Scand J Gastroenterol Suppl. 214:17–23. DOI: 10.3109/00365529609094509. PMID: 8722400.
20. Lee KJ. 2021; The usefulness of symptom-based subtypes of functional dyspepsia for predicting underlying pathophysiologic mechanisms and choosing appropriate therapeutic agents. J Neurogastroenterol Motil. 27:326–336. DOI: 10.5056/jnm21042. PMID: 34210898. PMCID: PMC8266502.
21. Moshiree B, Talley NJ. 2021; Functional dyspepsia: a critical appraisal of the European consensus from a global perspective. Neurogastroenterol Motil. 33:e14216. DOI: 10.1111/nmo.14216. PMID: 34337832.
22. Rothenberg ME, Cohen MB. 2007; An eosinophil hypothesis for functional dyspepsia. Clin Gastroenterol Hepatol. 5:1147–1148. DOI: 10.1016/j.cgh.2007.07.025. PMID: 17916543.
23. Gargala G, Lecleire S, François A, et al. 2007; Duodenal intraepithelial T lymphocytes in patients with functional dyspepsia. World J Gastroenterol. 13:2333–2338. DOI: 10.3748/wjg.v13.i16.2333. PMID: 17511033. PMCID: PMC4147143.
24. Liebregts T, Adam B, Bredack C, et al. 2011; Small bowel homing T cells are associated with symptoms and delayed gastric emptying in functional dyspepsia. Am J Gastroenterol. 106:1089–1098. DOI: 10.1038/ajg.2010.512. PMID: 21245834.
25. Lee MJ, Jung HK, Lee KE, Mun YC, Park S. 2019; Degranulated eosinophils contain more fine nerve fibers in the duodenal mucosa of patients with functional dyspepsia. J Neurogastroenterol Motil. 25:212–221. DOI: 10.5056/jnm18176. PMID: 30827070. PMCID: PMC6474707.
26. Järbrink-Sehgal ME, Sparkman J, Damron A, et al. 2021; Functional dyspepsia and duodenal eosinophil count and degranulation: a multiethnic US veteran cohort study. Dig Dis Sci. 66:3482–3489. DOI: 10.1007/s10620-020-06689-2. PMID: 33185786.
27. Yuan HP, Li XP, Yang WR, Li FK, Li YQ. 2015; Inducible nitric oxide synthase in the duodenal mucosa is associated with mast cell degranulation in patients with functional dyspepsia. Ann Clin Lab Sci. 45:522–527. PMID: 26586703.
28. Walker MM, Talley NJ, Prabhakar M, et al. 2009; Duodenal mastocytosis, eosinophilia and intraepithelial lymphocytosis as possible disease markers in the irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol Ther. 29:765–773. DOI: 10.1111/j.1365-2036.2009.03937.x. PMID: 19183150. PMCID: PMC4070654.
Fig. 1
Eosinophil and mast cell counts according to symptom-based subtypes of functional dyspepsia. Comparison of the eosinophil counts in the (A) duodenum, (B) stomach antrum, (C) stomach body and of mast cell counts, in the (D) duodenum, and (E) stomach antrum according to symptom-based subtypes of functional dyspepsia showed no significant difference among the groups. (F) The stomach body mast cell count was higher in the overlap group than in the EPS and PDS groups. EPS, epigastric pain syndrome; PDS, postprandial stress syndrome.
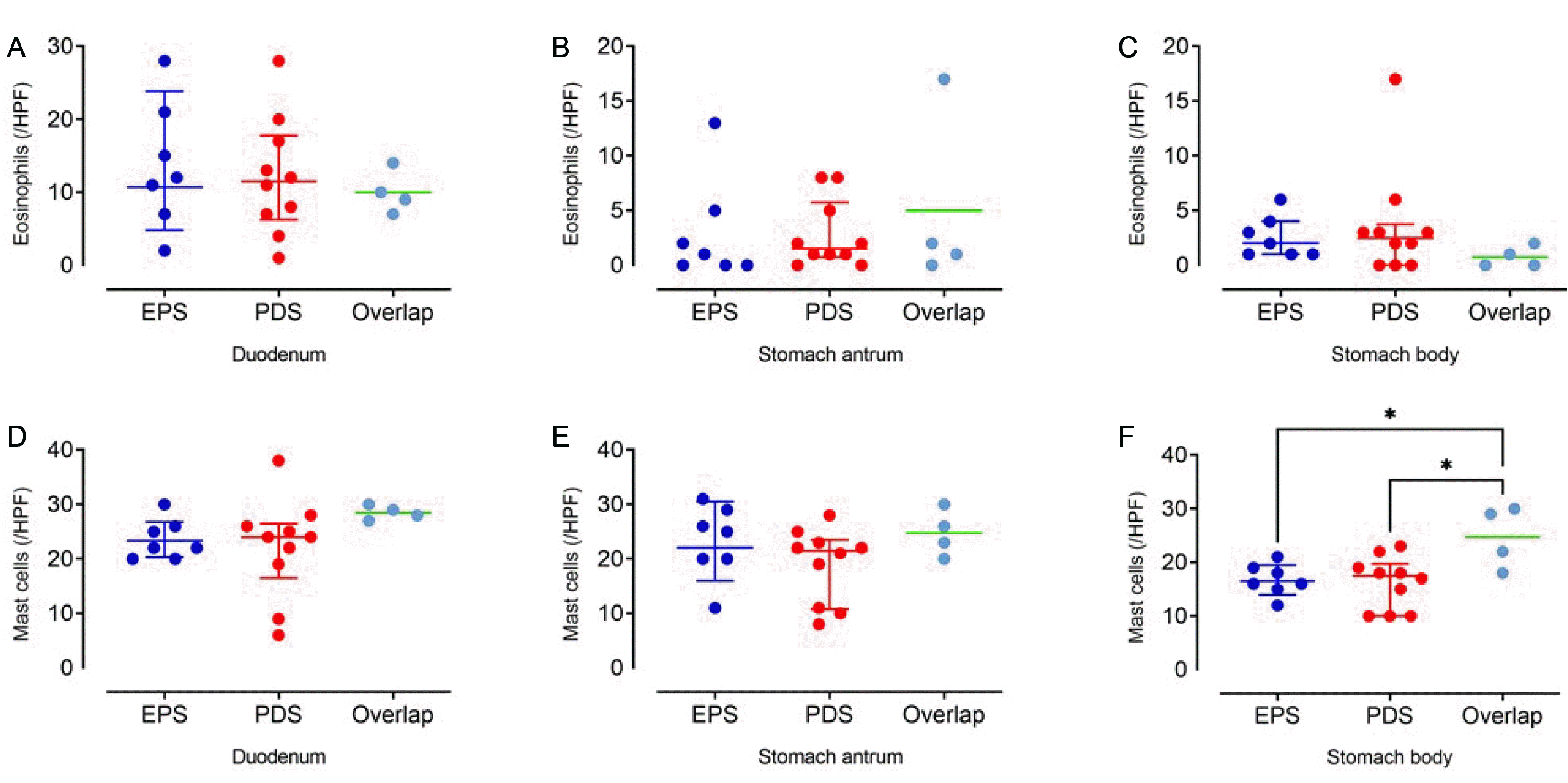
Table 1
Baseline Characteristics of the Study Participants according to the Symptom-based Subtypes of Functional Dyspepsia
Table 2
Stomach Pathologic Characteristics of the Study Participants according to Symptom-based Subtypes of Functional Dyspepsia
Table 3
Eosinophil and Mast Cell Counts according to the Symptom-based Subtypes of Functional Dyspepsia




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download