Abstract
Objective
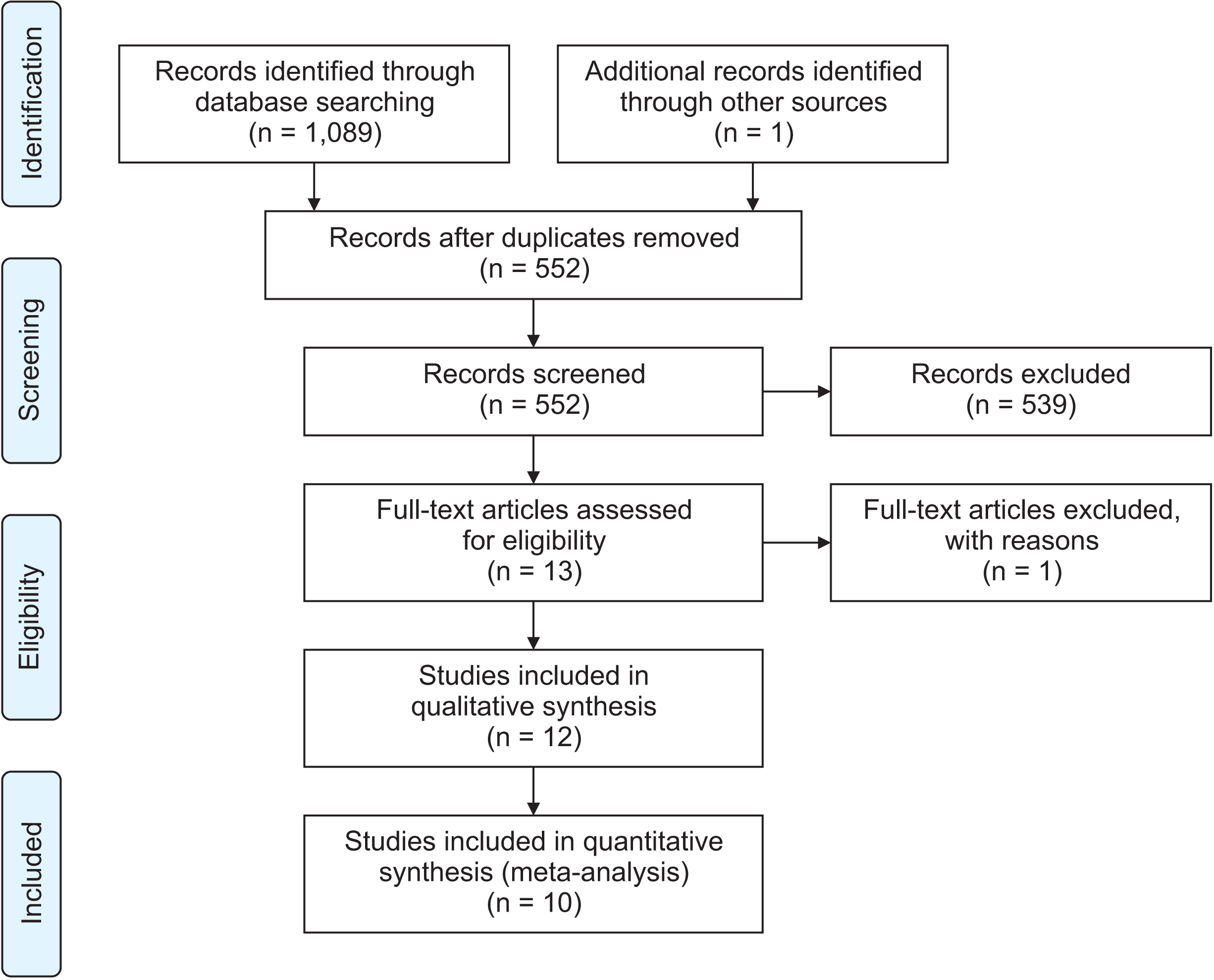
Methods
Results
ACKNOWLEDGEMENTS
REFERENCES
Figure 2
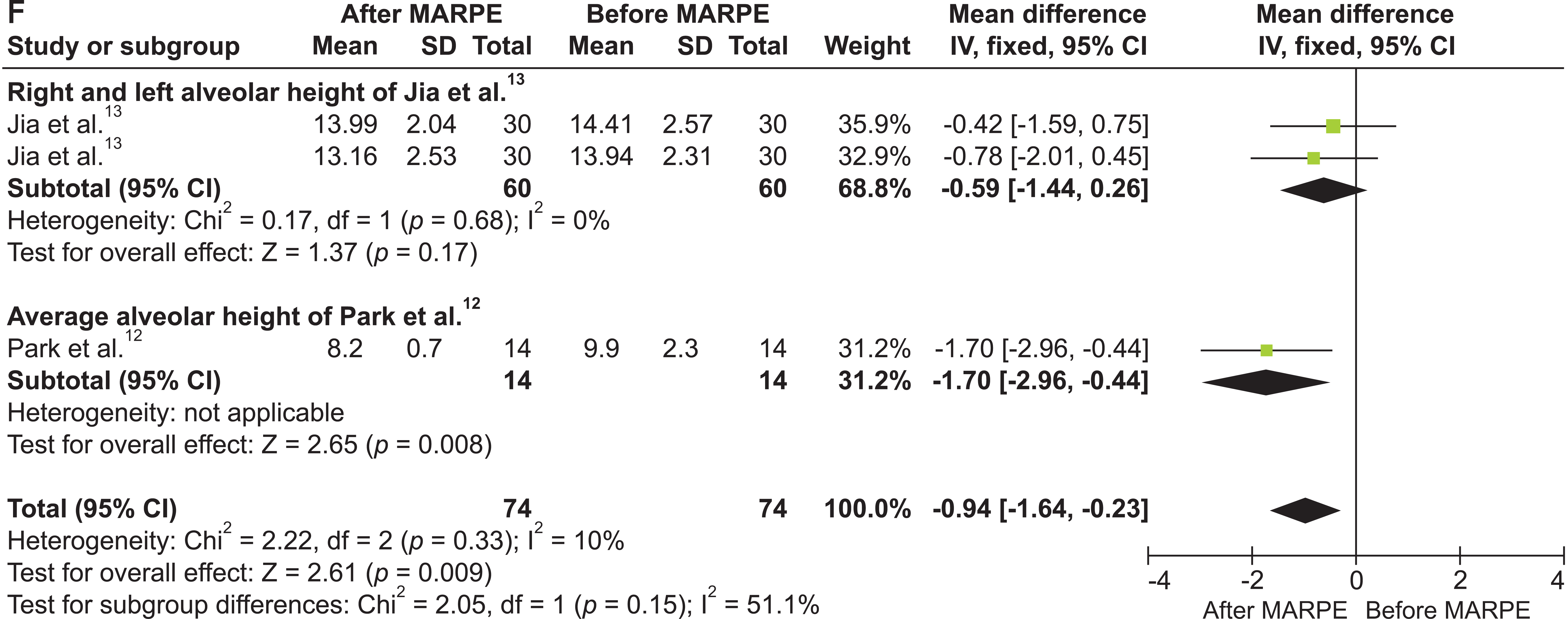
Table 1
Table 2
| Author | Year | Design | Group | Size | Sex (male/female) | Average age (yr) | Appliance | Activation protocol | Retention time | Measurement methods | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lagravère et al.18 | 2010 | >RCT | >MARPE | 21 | >8/13 | 14.24 ± 1.32 | >Bone-borne | >0.25 mm/d | >6 months | >CBCT | >6 months, 1 year |
| >RPE | 20 | >5/15 | 14.05 ± 1.35 | >Hyrax expander | >0.5 mm/d | >6 months | |||||
| >Control | 21 | >6/15 | 12.86 ± 1.19 | — | — | — | |||||
| Lin et al.17 | 2015 | >NRCT | >MARPE | 15 | >0/15 | 18.1 ± 4.4 | >C-expander | >0.25 mm/d | — | >CBCT | >3 months |
| >RPE | 13 | >0/13 | 17.4 ± 3.4 | >Hyrax expander | |||||||
| Yılmaz et al.15 | 2015 | >NRCT | >MARME | 14 | >8/6 | 13.2 ± 2.1 | >Bone-borne expander | 0.4 mm/d for first 7–10 d, 0.2 mm/3 d after the opening of the suture | — | Cephalograms, posteroanterior radiographs, dental casts | — |
| >Bonded RME | 14 | >8/6 | 12.1 ± 2.1 | >Bonded expander | |||||||
| >Banded RME | 14 | >6/8 | 13.4 ± 1.7 | >Banded expander | |||||||
| Akin et al.19 | 2016 | >NRCT | >MARPE | 9 | >5/4 | 13.61 ± 0.72 | >Hybrid expander | 0.5 mm/d for the first week, then 0.25 mm/d for 3 weeks | — | >CBCT | — |
| Choi et al.8 | 2016 | >NRCT | >MARPE | 20 | >10/10 | 20.9 ± 2.9 | >Bone-borne expander | >0.2 mm/2 d | >3 months | Posteroanterior cephalograms | >30.2 ± 13.2 months |
| Cantarella et al.16 | 2017 | >NRCT | >MARPE | 15 | >6/9 | 17.2 ± 4.2 | Maxillary skeletal expander | 0.5 mm/d at first, then 0.25 mm/d after inter-incisal diastema appeared | >More than 3 months | >CBCT | — |
| Lim et al.10 | 2017 | >NRCT | >MARPE | 24 | >8/16 | 21.6 ± 3.1 | >Bone-borne expander | >0.2 mm/d | >4 months | >CBCT | 1 year (14.17 ± 2.70 month) |
| Park et al.12 | 2017 | >NRCT | >MARPE | 14 | >9/5 | 20.1 ± 2.4 | >Bone-borne expander | >0.2 mm/d | — | >CBCT | — |
| Celenk-Koca et al.20 | 2018 | >RCT |
>Conventional- RME |
20 | >8/12 | 13.84 ± 1.36 | >Hyrax expander | >2 turns/d | — | >CBCT | — |
| >MARPE | 20 | >7/13 | 13.81 ± 1.23 | Miniscrew-support expander | |||||||
| Ngan et al.14 | 2018 | >NRCT | >MARPE | 8 | >6/2 | 21.9 ± 1.5 | Maxillary skeletal expander | Varied with the severity of transverse discrepancy | — | >CBCT | — |
| Oliveira et al.11 | 2020 | >NRCT | >MARPE | 28 | >10/18 | 15–37 | Miniscrew-support expander | >2/4 turn/d | >4 months | >CBCT | >6 months |
| Jia et al.13 | 2021 | >RCT | >MARPE | 30 | >9/21 | 14.8 ± 1.5 | >Hyrax expander | >0.5 mm/d | >3 months | >CBCT | — |
| >Hyrax | 30 | >12/18 | 15.1 ± 1.6 | Four-point MARPE expander |
Table 3
| Author | Year | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other sources of bias | Overall bias |
|---|---|---|---|---|---|---|---|---|---|
| Lagravère et al.18 | 2010 | Low risk | Unclear | Low risk | Unclear | Low risk | Unclear | Low risk | Low risk |
|
Celenk-Koca et al.20 |
2018 | Low risk | Low risk | Low risk | Low risk | Low risk | Unclear | Low risk | Low risk |
| Jia et al.13 | 2021 | Low risk | Low risk | Low risk | Unclear | Low risk | Unclear | Low risk | Low risk |
Table 4
| Author | Year | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lin et al.17 | 2015 | 2 | 1 | 2 | 2 | 1 | 1 | 2 | 0 | 2 | 1 | 1 | 2 | 17 |
| Yılmaz et al.15 | 2015 | 2 | 1 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 21 |
| Akin et al.19 | 2016 | 2 | 1 | 2 | 2 | 0 | 0 | 2 | 0 | 9 | ||||
| Choi et al.8 | 2016 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 2 | 12 | ||||
| Cantarella et al.16 | 2017 | 2 | 2 | 2 | 2 | 0 | 0 | 2 | 0 | 10 | ||||
| Lim et al.10 | 2017 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 14 | ||||
| Park et al.12 | 2017 | 2 | 2 | 2 | 2 | 0 | 0 | 2 | 0 | 10 | ||||
| Ngan et al.14 | 2018 | 2 | 2 | 2 | 2 | 0 | 0 | 2 | 0 | 10 | ||||
| Oliveira et al.11 | 2020 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 14 |
Items 1–12 represent the following: (1) a clearly stated aim; (2) inclusion criteria for consecutive patients; (3) prospective collection of data; (4) endpoints appropriate for the aim of this study; (5) unbiased assessment of the study endpoint; (6) follow-up period appropriate to the aim of the study; (7) loss to follow-up of less than 5%; (8) prospective calculation of study size; (9) adequate control group; (10) contemporary group; (11) baseline equivalence of groups; and (12) appropriate statistical analyses. A score of 0 means not mentioned, 1 means reported but inadequate, and 2 means reported and adequate. The total score is 24 for studies with control groups and 16 for studies without control groups. Quality is considered low (0–9 for studies with control groups, 0–7 for studies without control groups), moderate (10–20 for studies with control groups, 8–13 for studies without control groups), or high (20–24 for studies with control groups, 14–16 for studies without control groups) based on the total score.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download