Abstract
Donations from deceased donors have been increasing since the introduction of expanded criteria for donor kidney selection. Several studies have shown that patients receiving deceased donor kidneys using these expanded criteria have improved survival compared to those remaining on the waiting list during hemodialysis. It is important, however, to consider that some of the kidneys classed as usable under the expanded criteria may in fact be unacceptable. To address this concern, preoperative biopsy and imaging of deceased donor kidneys are increasingly being used to assess candidate kidneys. We present the case of a 44-year-old female patient who underwent deceased donor kidney transplantation with negative complement-dependent cytotoxicity and flow cytometry crossmatch. Hours after graft reperfusion, given clinical evidence of primary nonfunction in the kidney, the patient underwent nephrectomy. Despite negative tests for blood type difference and crossmatch, and although the main artery and vein were well perfused, the kidney graft was never functional, and pathologic findings showed thrombotic microangiopathy and diffuse acute tubular necrosis. We conclude that further work on ideal criteria for identifying acceptable donor kidneys is needed.
Go to : 
Go to : 
Donations from deceased donors have been increasing since the introduction of expanded criteria for donor kidney selection. Even with this increase, however, the available organ pool is still not able to cover current shortages. Efforts are being made to expand the donor pool to include donations from brain-dead individuals after cardiac arrest [1]. According to several studies, identifying and transplanting deceased donor kidneys using the expanded criteria results in survival gains compared to outcomes for patients on the waiting list with hemodialysis [2]. It is important, however, to consider that some kidneys selected under the expanded criteria may turn out to be unacceptable [3]. To address this concern, preoperative biopsy and imaging of deceased donor kidneys are increasingly being used to assess candidate kidneys [4]. Due to increased knowledge about transplant immunology and the development of serologic tests, hyperacute rejection has become extremely rare. Nevertheless, cases of early graft loss (EGL) continue to occur [3].
We report a case of intraoperative graftectomy due to primary non-function in a deceased donor kidney transplantation (DDKT) preoperatively confirmed to be human leukocyte antigen (HLA) crossmatch-negative by both complement-dependent cytotoxicity (CDC) and flowcytometry.
Go to : 
This study was approved by the Institutional Review Board of Samsung Medical Center (IRB No. 2022-04-124). As this study was a retrospective study using medical records without using personally identifiable information, consent for research was exempted.
A 44-year-old East Asian female patient presented with end-stage renal disease due to chronic glomerulonephritis. The patient had malignant hypertension and had been undergoing hemodialysis for the past 12 years. She underwent DDKT in July 2021.
The donor was a 25-year-old woman with no underlying disease such as diabetes mellitus, hypertension, renal disease, or hematologic disease; instead, she had experienced traumatic subdural hemorrhage due to a fall. At the time of admission, the creatinine level was 1.4 mg/dL, the international normalized ratio (INR) was 1.84, and the urine output was sufficient. The fresh frozen plasma transfusion amount was 3–4 pints per day before donor organ harvesting, and the INR was maintained between 1.3 and 1.4. An 8-pint platelet transfusion was also performed on both the day of initial hospitalization and the day of the operation. At the time of the first electroencephalography examination 2 days later, the creatinine level had risen to 4 mg/dL, and the urine output had rapidly decreased to a total of 700 mL per day. It was decided to proceed with continuous renal replacement therapy, as this was 36 hours before the transplant. No other medical problems were observed by ultrasound (US) on the day of the organ harvesting operation, but hyperechoic renal parenchyma with hypoechoic pyramids was observed in both kidneys.
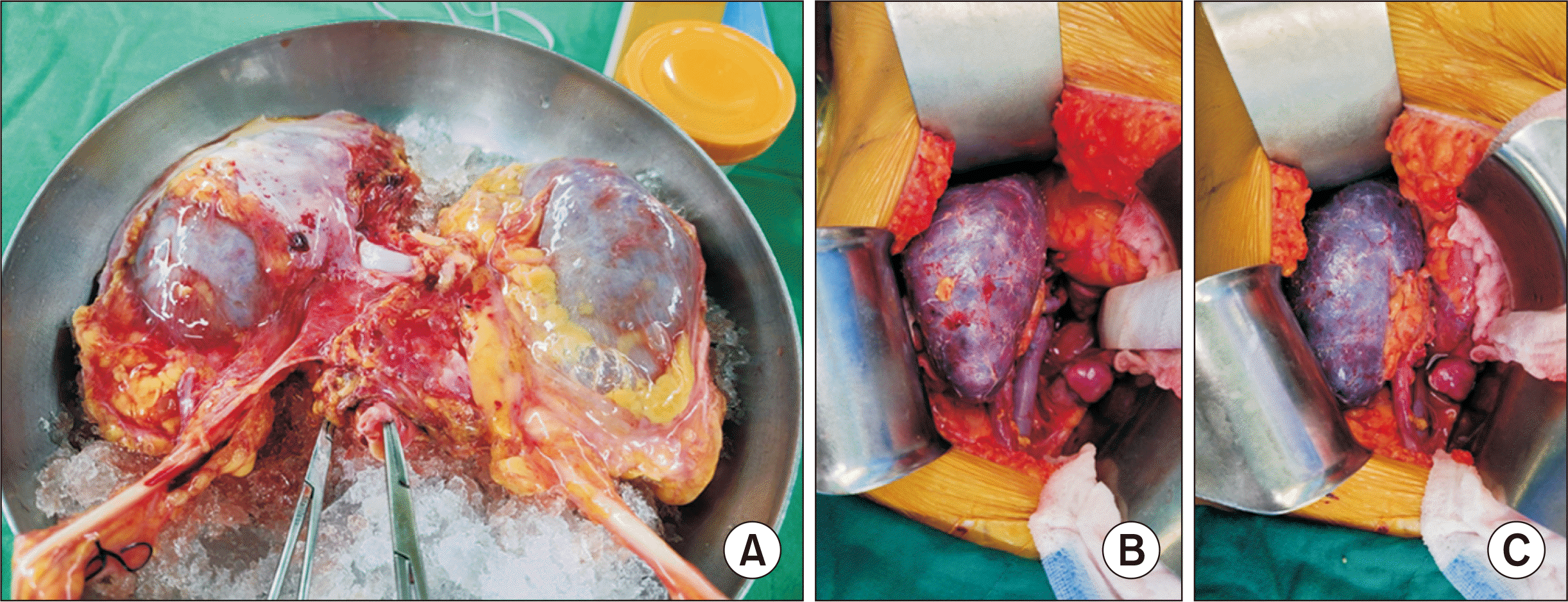
HLA mismatch results of both class I and II were panel reactive antibody 0%. Although the scores for the Kidney Donor Profile Index (KDPI) of 39% and Kidney Donor Risk Index (KDRI) of 0.90 were acceptable, the intraoperative donor kidney status after perfusion was marginal, with the kidneys exhibiting grossly abnormal bruised coloration with diffuse petechiae and mild swelling (Fig. 1A).
Kidney transplantation was performed via anastomosis of the graft renal vein and the recipient's external iliac vein with the graft renal artery and the recipient's external iliac artery in an end-to-side method. The cold ischemia time (CIT) was about 6 hours, and the warm ischemia time was 78 minutes. Warm saline irrigation was performed for about 1 hour since urination did not occur immediately, and the purple kidney color persisted after reperfusion (Fig. 1B and C). Blood flow of the main renal vessels was measured using intraoperative renal Doppler and peripheral blood flow with the resistive index (RI) value. Both the main renal artery and vein were patent, but the peripheral flow of the graft kidney was very weak, and the RI value was greater than 0.9. For these reasons, an immediate graftectomy was performed.
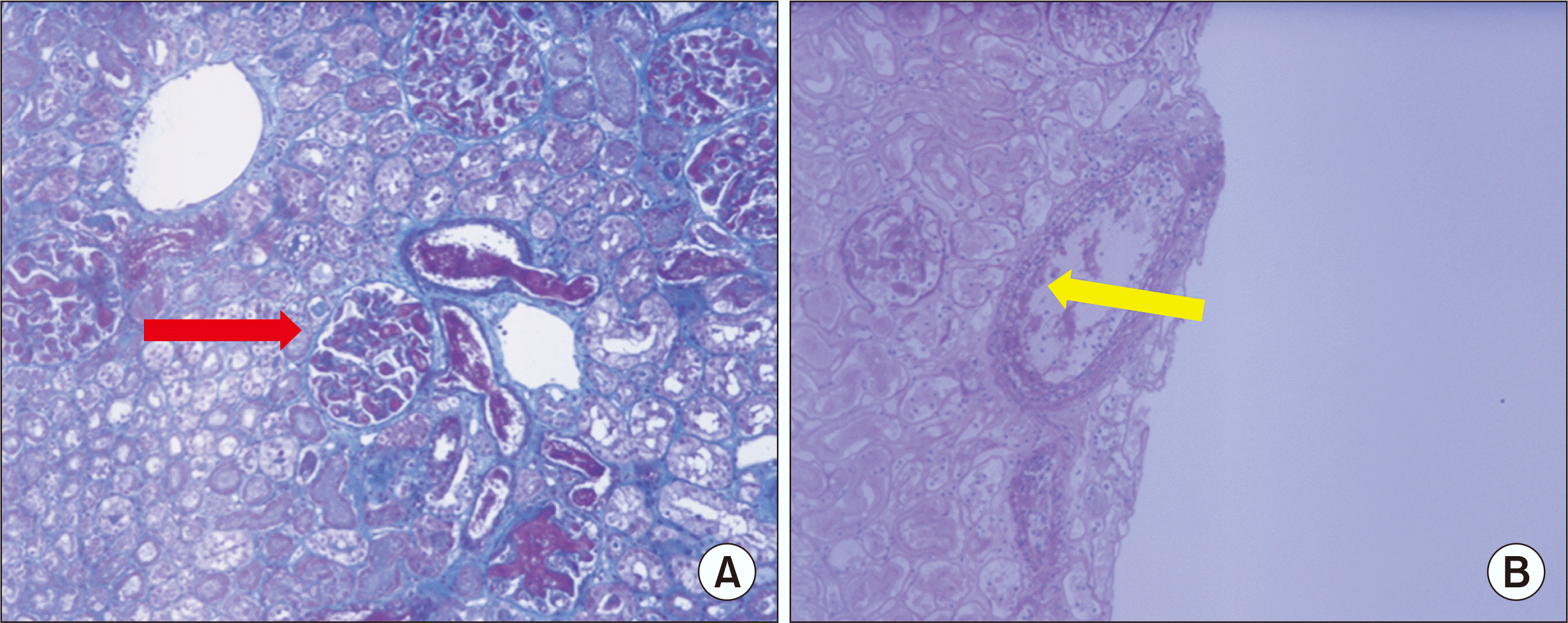
As a matter of routine, zero-time biopsies of the deceased donor kidneys were performed immediately after reperfusion and compared to the pathologic findings of the entire kidney. Sections showed fragmented cores of renal cortex containing 16 glomeruli, none of which were globally sclerotic. The capillary loops were diffusely expanded and contained fibrinous material, while the tubules revealed acute damage and minimal atrophy accompanied by minimal interstitial fibrosis and minimal mononuclear infiltration. The arteries exhibited endothelial inflammation with fibrin deposition. Microscopic findings of zero-time biopsy using Masson's trichrome method demonstrated a thrombus filling the glomerulus (Fig. 2A), and periodic acid–Schiff staining revealed endothelial inflammation in the arteries (Fig. 2B). The microscopic findings shown in Fig. 2 suggest thrombotic microangiopathy (TMA) and diffuse acute tubular necrosis.
The pathologic findings for the entire specimen were not significantly different from the results reported above. The glomeruli were slightly increased in size and normocellular. The mesangial matrix was not increased. The capillary loops were diffusely expanded and contained fibrinous material. The tubules revealed acute damage and minimal atrophy accompanied by minimal interstitial fibrosis and minimal mononuclear infiltration. The arteries exhibited endothelial inflammation with fibrin deposition.
Go to : 
Starting with the patient’s ABO blood type, we assessed HLA crossmatch using both complement-dependent cytotoxicity and flow cytometry and single identification of HLA type. To minimize graft failure due to donor factors, we considered the KDPI and KDRI values. Given the kidney pathologic findings shown in the zero-time biopsy and the donor kidney after perfusion, we concluded that this expanded criteria donor (ECD) kidney was not acceptable for transplantation and resulted in graft failure.
The pathologic findings can be summarized as TMA, diffuse acute tubular necrosis, and endothelial inflammation. TMA, a pathologic description, can manifest in a diverse range of conditions and presentations, with acute kidney injury a common and prominent cause due to an observed propensity of the glomerular circulation for endothelial damage and occlusion [5]. We interpret the pathologic findings, therefore, as an extremely injured kidney rather than hyperacute rejection.
Given the current ongoing scarcity of organs, transplant centers are continually weighing the risks of adverse posttransplantation outcomes, such as EGL, against the long-term implications for the patient. EGL is associated with significant negative consequences including profound short- and long-term mortality rate increases, reduced chances of relisting and retransplantation, and increased risk of recurrent EGL for those undergoing retransplantation [6]. For these reasons, it is important to identify acceptable kidneys among ECDs.
A recent study evaluated renal cortical volume using computed tomography (CT) to predict graft function in living donor kidney transplants, although that was not necessarily correlated with graft survival [7,8]. Another study showed that US measurements, especially cortical thickness, were good for evaluating renal function [9]. US was more useful than CT for evaluating the state of a kidney in a deceased donor, since most deceased donors sustain acute kidney injury [10] and cannot be transferred promptly from an intensive care unit to a CT room.
Donor kidney biopsy findings have been debated as an independent predictor of donor quality beyond other clinical indices. Donor kidney biopsies and their associated donor characteristics were analyzed, and unfavorable pathologic findings were found to be associated with an increased incidence of delayed graft function and poor outcomes for transplanted kidneys [11,12]. While donor biopsy findings therefore appear to have some predictive use, it is unclear to what extent they improve outcomes over what donor age and function are already able to reveal. There are also several challenges with donor kidney biopsy [13]. These include the fact that frozen sections are not appropriate—and formalin fixation therefore adds several hours to cold ischemia time—and that an on-call pathologist must be available. In a case like this one, however, if the kidney status could have been determined through a frozen biopsy before perfusion, this devastating result might have been avoided.
In conclusion, this is a case report of immediate graftectomy due to primary non-function. It is an important example of how early graft failure can occur even when active preoperative evaluations are performed on both donors and recipients. This report is not intended to reduce the use of kidneys from ECDs, but rather to help determine which ECD kidneys are truly acceptable through various methods suggested above.
Go to : 
ACKNOWLEDGMENTS
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Funding/Support
This study was supported by research grant from the Korean Society for Transplantation (2022-00-03001-013).
Author Contributions
Conceptualization: KWL, JBP. Data curation: KWL. Project adminitration: KWL. Writing–original draft: SOY, MJK. Writing–review & editing: SOY, KWL, JBP.
Go to : 
REFERENCES
1. Dahmen M, Becker F, Pavenstädt H, Suwelack B, Schütte-Nütgen K, Reuter S. 2019; Validation of the Kidney Donor Profile Index (KDPI) to assess a deceased donor's kidneys' outcome in a European cohort. Sci Rep. 9:11234. DOI: 10.1038/s41598-019-47772-7. PMID: 31375750. PMCID: PMC6677881.
2. Heldal K, Hartmann A, Grootendorst DC, de Jager DJ, Leivestad T, Foss A, et al. 2010; Benefit of kidney transplantation beyond 70 years of age. Nephrol Dial Transplant. 25:1680–7. DOI: 10.1093/ndt/gfp681. PMID: 20038521. PMCID: PMC2856560.
3. Swinarska JT, Stratta RJ, Rogers J, Chang A, Farney AC, Orlando G, et al. 2021; Early graft loss after deceased-donor kidney transplantation: what are the consequences? J Am Coll Surg. 232:493–502. DOI: 10.1016/j.jamcollsurg.2020.12.005. PMID: 33348013.
4. Stenberg B, Talbot D, Khurram M, Kanwar A, Ray C, Mownah O, et al. 2011; A new technique for assessing renal transplant perfusion preoperatively using contrast-enhanced ultrasound (CEUS) and three-dimensional ultrasound (3DUS): a porcine model pilot study. Ultraschall Med. 32 Suppl 2:E8–13. DOI: 10.1055/s-0031-1281650. PMID: 22179806.
5. Kerr H, Richards A. 2012; Complement-mediated injury and protection of endothelium: lessons from atypical haemolytic uraemic syndrome. Immunobiology. 217:195–203. DOI: 10.1016/j.imbio.2011.07.028. PMID: 21855165. PMCID: PMC4083254.
6. de Kok MJ, Schaapherder AF, Mensink JW, de Vries AP, Reinders ME, Konijn C, et al. 2020; A nationwide evaluation of deceased donor kidney transplantation indicates detrimental consequences of early graft loss. Kidney Int. 97:1243–52. DOI: 10.1016/j.kint.2020.01.043. PMID: 32359810.
7. Juluru K, Rotman JA, Masi P, Spandorfer R, Ceraolo CA, Giambrone AE, et al. 2015; Semiautomated CT-based quantification of donor kidney volume applied to a predictive model of outcomes in renal transplantation. AJR Am J Roentgenol. 204:W566–72. DOI: 10.2214/AJR.14.13454. PMID: 25905963.
8. Sikora MB, Shaaban A, Beddhu S, Bourija H, Wei G, Baird B, et al. 2012; Effect of donor kidney volume on recipient outcome: does the "dose" matter? Transplantation. 94:1124–30. DOI: 10.1097/TP.0b013e31826f135e. PMID: 23060282.
9. Beland MD, Walle NL, Machan JT, Cronan JJ. 2010; Renal cortical thickness measured at ultrasound: is it better than renal length as an indicator of renal function in chronic kidney disease? AJR Am J Roentgenol. 195:W146–9. DOI: 10.2214/AJR.09.4104. PMID: 20651174.
10. Fung A, Zhao H, Yang B, Lian Q, Ma D. 2016; Ischaemic and inflammatory injury in renal graft from brain death donation: an update review. J Anesth. 30:307–16. DOI: 10.1007/s00540-015-2120-y. PMID: 26746399.
11. De Vusser K, Lerut E, Kuypers D, Vanrenterghem Y, Jochmans I, Monbaliu D, et al. 2013; The predictive value of kidney allograft baseline biopsies for long-term graft survival. J Am Soc Nephrol. 24:1913–23. DOI: 10.1681/ASN.2012111081. PMID: 23949799. PMCID: PMC3810080.
12. Gaber LW, Moore LW, Alloway RR, Amiri MH, Vera SR, Gaber AO. 1995; Glomerulosclerosis as a determinant of posttransplant function of older donor renal allografts. Transplantation. 60:334–9. DOI: 10.1097/00007890-199508270-00006. PMID: 7652761.
13. Dare AJ, Pettigrew GJ, Saeb-Parsy K. 2014; Preoperative assessment of the deceased-donor kidney: from macroscopic appearance to molecular biomarkers. Transplantation. 97:797–807. DOI: 10.1097/01.TP.0000441361.34103.53. PMID: 24553618.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download