Abstract
Varicella zoster virus (VZV) infection can follow a virulent course, leading to possible infection-related mortality in immunocompromised hosts. Visceral disseminated VZV infection is a rare disease with a high mortality rate in immunocompromised patients. We present a case of acute liver failure and acute myocarditis due to visceral disseminated VZV infection in an immunocompromised patient who had recently received kidney transplantation and who subsequently showed dramatic improvement after treatment with intravenous acyclovir and intravenous immunoglobulin. Severe epigastric pain preceded the vesicular skin lesions; therefore, the diagnosis and treatment could have been delayed. Such delays have caused mortality in most previous cases. Therefore, it is necessary to consider visceral disseminated viral infection in the differential diagnosis of immunocompromised patients when multi-organ failure progresses with an unknown cause.
Varicella zoster virus (VZV) causes two clinical syndromes, namely varicella (chickenpox) and herpes zoster (shingles). Primary infection, which causes chickenpox, usually presents in childhood and is characterized by a maculopapular vesicular rash. The virus may infect the dorsal root ganglia and remain in a latent condition. Reactivation occurs in elderly adults and immunocompromised individuals whose cell-mediated immunity has declined, and reactivated VZV can cause complications such as postherpetic neuralgia. Disseminated herpes zoster is especially common in immunocompromised patients and occurs due to the active replication of VZV in other organs, such as the lungs, liver, or brain [1].
In this report, we present a case of disseminated VZV infection in an immunocompromised patient who had recently undergone kidney transplantation and was taking high-dose immunosuppressant medication. Although he was vaccinated against VZV during childhood, the infection occurred and ultimately caused fulminant hepatitis and acute myocarditis. Fortunately, the patient recovered from acute liver failure and myocarditis following simultaneous administration of acyclovir and intravenous immunoglobulin.
This study was approved by the Institutional Review Board of Seoul National University Hospital (IRB No. H-2109-092-1255), and informed consent was obtained from the patient.
A 39-year-old male patient, who had undergone kidney transplantation 3 weeks earlier, presented to the emergency department of our hospital with abrupt abdominal pain. He had undergone a kidney transplant for end-stage renal disease (ESRD) due to immunoglobulin A nephropathy and had been on peritoneal dialysis for 3 years prior to the transplant. The donor was the patient’s wife, who was ABO-compatible but had four human leukocyte antigen (HLA) mismatches. No preformed donor-specific HLA antibodies were detected in the patient. He was primarily treated with an interleukin-2 receptor blocking monoclonal antibody (basiliximab) to induce immunosuppression. The subsequent maintenance immunosuppressive treatment consisted of prednisolone, tacrolimus, and mycophenolate mofetil. Since the graft functions were slow, an indication allograft biopsy was performed on the ninth day posttransplant and showed a T cell-mediated acute rejection, grade 1A. The patient was then treated with 3 g of methylprednisolone pulse therapy.
Upon physical examination and from his reported history, it was evident that the patient had a squeezing pain in the epigastric area which worsened during movement and improved with rest. There was tenderness in the epigastric area, but rebound tenderness was absent; however, his abdominal pain was aggravated in the supine position. Blood tests performed in the emergency room showed slightly increased levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total bilirubin, but the C-reactive protein level was in the normal range. Esophagogastroduodenoscopy and computed tomography (CT) scans of the abdomen and pelvis showed no abnormal findings other than erosive gastritis, a suspected cause of the abdominal pain. Apart from the abdominal pain, there were no other findings, such as a maculopapular vesicular rash.
Gradually, the patient’s abdominal pain worsened. Additionally, he complained of tachycardia and chest pain. Further blood tests did not show leukocytosis or increased C-reactive protein levels, but the AST, ALT, and total bilirubin levels were significantly elevated. The initial AST, ALT, and total bilirubin were 314 U/L, 384 U/L, and 1.4 mg/dL, but these parameters gradually increased to peak values of 5,276 U/L, 4,776 U/L, and 12.0 mg/dL, respectively. The C-reactive protein levels remained within the normal range, but vancomycin-resistant Enterococcus was detected in the blood culture. An electrocardiogram showed sinus tachycardia with non-specific ST changes, and cardiac enzymes (creatine kinase-myocardial band and troponin I) were elevated. Echocardiography showed a decreased ejection fraction of 40%, indicating global hypokinesia. Before surgery, there had been no regional wall motion abnormality and the ejection fraction had been 60%. A serological test was performed to determine whether the patient’s hepatitis and myocarditis were caused by a viral infection. He tested negative for hepatitis A virus immunoglobulin G antibody, hepatitis A virus immunoglobulin M antibody, hepatitis B surface antigen, anti-hepatitis C virus, and VZV immunoglobulin M antibody; however, the tests for VZV immunoglobulin G antibody and human herpesvirus 6 were positive.
Three days after the onset of abdominal pain, several blisters appeared on his face, chin, and the anterior portion of his chest. Macroscopically, these appeared to be furuncles, but vesicle aspiration and subsequent vesicular fluid culture were performed to differentiate herpes zoster. The aspirated vesicle fluid tested negative on the Tzanck smear test, but both the vesicular fluid and the patient’s blood tested positive for the VZV polymerase chain reaction analysis (Table 1).
Since there were significant increases in the parameters of the liver function tests, a liver biopsy was performed and the results revealed multifocal, zonal, coagulative, parenchymal necrosis. The liver tissue specimens tested negative for herpes simplex virus, cytomegalovirus, and adenovirus immunohistochemistry. However, VZV immunohistochemistry and molecular pathology tests were not performed because the test method was not available in our hospital. Although viral inclusions were not observed in the liver tissue specimens, the distribution of tissue necrosis pointed preferentially towards viral hepatitis.
Based on the above observations, the patient was diagnosed with fulminant hepatitis and acute myocarditis resulting from disseminated herpes zoster. Accordingly, acyclovir (10 mg/kg twice daily; renal dosing) was administered immediately after blisters were found. Despite administration of acyclovir, the AST, ALT, and total bilirubin levels continued to increase. Therefore, after biopsy confirmation, 1 g of intravenous immunoglobulin was additionally administered. The patient’s AST, ALT, and total bilirubin levels decreased for the first time the following day (Fig. 1) and his general condition and abdominal pain also improved. The cardiac enzyme levels had also decreased, the sinus tachycardia was converted to normal sinus rhythm, and improved global hypokinesia with an ejection fraction of 60% was seen on the follow-up echocardiography.
In contrast, the patient’s renal function was deteriorating gradually, with the creatinine level increasing from 1.8 mg/dL initially to 4.5 mg/dL. Moreover, schistocytes were confirmed by peripheral blood smears, along with a decreased haptoglobin level and thrombocytopenia. Therefore, renal biopsy was performed and confirmed focal thrombotic microangiopathy, suspicious for acute T cell-mediated rejection and suggestive of acute calcineurin inhibitor toxicity. ADAMTS-13 activity was 96%. To treat the thrombotic microangiopathy, therapeutic plasmapheresis and intravenous immunoglobulin (0.5 g/kg) were simultaneously performed six times every other day. On the day after the last plasmapheresis, rituximab (375 mg/m2) was administered and his renal function gradually improved. As his symptoms improved, he was discharged from the hospital and follow-up visits were conducted at an outpatient clinic.
Fulminant hepatitis and acute myocarditis occurred due to visceral disseminated herpes zoster infection in a 39-year-old ESRD patient who had undergone kidney transplantation. His history revealed that he was vaccinated for zoster in childhood and that he had not had recent exposure to anyone with symptoms of VZV infection. Since VZV immunoglobulin G antibody was positive while VZV immunoglobulin M was negative, it was more likely a VZV reactivation. Reactivation commonly occurs in elderly adults and immunocompromised patients because the T cell-related immune response weakens with aging or administration of chemotherapy or immunosuppressive drugs. T cell-related immunity plays an important role in protection against exogenous VZV exposure and endogenous VZV reactivation [2]. When the T cell-related immune response weakens, the individual becomes vulnerable to VZV re-exposure or latent VZV reactivation, both of which present as herpes zoster. Due to posttransplantation immunosuppressant administration and steroid pulse therapy for 2 weeks after surgery, the patient’s T cell immunity had decreased and he was immunocompromised. Therefore, it is highly probable that the latent VZV reactivation occurred due to the suppression of T cell immunity. Moreover, the occurrence of vancomycin-resistant Enterococcus bacteremia was also related to the decrease in immunity.
Previous studies reported that, in cases of VZV infection in immunocompromised patients, the possibility of the infection disseminating to internal organs and causing pneumonia, hepatitis, or meningitis is higher than the chance of skin lesions appearing, leading to increased mortality in such patients [3]. To date, nine cases of acute hepatic failure and acute myocarditis caused by disseminated herpes zoster have been reported, of which three survived and six died [4-12]. In most cases, the patients initially complained of epigastric pain, but there were no specific findings in the blood tests or on CT scans when the abdominal pain first occurred [13,14]. Skin lesions occurred within three to four days of the onset of abdominal pain, and blood tests performed at that time confirmed acute liver failure. Since, in most of the cases, acyclovir was administered after the blisters were confirmed to be a herpes zoster infection, it was already too late to prevent progression to fulminant hepatitis [5-7,9-12].
Fortunately, contrary to the previous reports, the current patient’s condition improved from the disseminated zoster with visceral organ involvement and he survived. This case differed from the other cases in that, when a vesicle was found, a test was conducted with the possibility of disseminated viral infection in mind, and acyclovir was preemptively administered before confirming the results. In addition, a quick and active examination was conducted and intravenous immunoglobulin was administered when acyclovir showed no therapeutic effect.
In conclusion, even if a patient has been vaccinated against several viruses, viral infections which are known to be disseminated and invade visceral organs should be included in the differential diagnosis when multi-organ failure progresses with an unknown cause. In addition, visceral disseminated zoster infection progresses rapidly, making it important not to delay treatment.
ACKNOWLEDGMENTS
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: HL. Data curation: all authors. Project administration: HL. Writing–original draft: HL, JP. Writing–review & editing: all authors.
REFERENCES
1. Jameson JL, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J. 2018. Harrison's principles of internal medicine, 20e. McGraw Hill;New York, NY:
2. Grose C, Enquist LW. 2020; The round trip model for severe herpes zoster caused by live attenuated varicella vaccine virus. J Med Virol. 92:938–40. DOI: 10.1002/jmv.25664. PMID: 31943220. PMCID: PMC7354881.
3. Ndumbe PM, Cradock-Watson J, Levinsky RJ. 1988; Natural and artificial immunity to varicella zoster virus. J Med Virol. 25:171–8. DOI: 10.1002/jmv.1890250207. PMID: 2839610.
4. Alvite-Canosa M, Paniagua-Martín MJ, Quintela-Fandiño J, Otero A, Crespo-Leiro MG. 2009; Fulminant hepatic failure due to varicella zoster in a heart transplant patient: successful liver transplant. J Heart Lung Transplant. 28:1215–6. DOI: 10.1016/j.healun.2009.06.017. PMID: 19782606.
5. Azak E, Cetin II. 2020; Acute myocarditis following varicella zoster infection in an immunocompetent adolescent: an uncommon complication. Arch Argent Pediatr. 118:e284–7. DOI: 10.5546/aap.2020.eng.e284.
6. Brewer EC, Hunter L. 2018; Acute liver failure due to disseminated varicella zoster infection. Case Reports Hepatol. 2018:1269340. DOI: 10.1155/2018/1269340. PMID: 30363707. PMCID: PMC6180957.
7. Dits H, Frans E, Wilmer A, Van Ranst M, Fevery J, Bobbaers H. 1998; Varicella-zoster virus infection associated with acute liver failure. Clin Infect Dis. 27:209–10. DOI: 10.1086/514613. PMID: 9675478.
8. Lechiche C, Le Moing V, François Perrigault P, Reynes J. 2006; Fulminant varicella hepatitis in a human immunodeficiency virus infected patient: case report and review of the literature. Scand J Infect Dis. 38:929–31. DOI: 10.1080/00365540600561785. PMID: 17008242.
9. Maggi U, Russo R, Conte G, Chiumello D, Lunghi G, Maggioni M, et al. 2011; Fulminant multiorgan failure due to varicella zoster virus and HHV6 in an immunocompetent adult patient, and anhepatia. Transplant Proc. 43:1184–6. DOI: 10.1016/j.transproceed.2011.01.142. PMID: 21620083.
10. Natoli S, Ciotti M, Paba P, Testore GP, Palmieri G, Orlandi A, et al. 2006; A novel mutation of varicella-zoster virus associated to fatal hepatitis. J Clin Virol. 37:72–4. DOI: 10.1016/j.jcv.2006.06.004. PMID: 16876475.
11. Saitoh H, Takahashi N, Nanjo H, Kawabata Y, Hirokawa M, Sawada K. 2013; Varicella-zoster virus-associated fulminant hepatitis following allogeneic hematopoietic stem cell transplantation for multiple myeloma. Intern Med. 52:1727–30. DOI: 10.2169/internalmedicine.52.0118. PMID: 23903507.
12. Toffaha A, El Ansari W, Ramzee AF, Afana M, Aljohary H. 2019; Rare presentation of primary varicella zoster as fatal fulminant hepatitis in adult on low-dose, short-term steroid: case report. Ann Med Surg (Lond). 48:115–7. DOI: 10.1016/j.amsu.2019.10.034. PMID: 31763037. PMCID: PMC6864176.
13. Amaral V, Shi JZ, Tsang AM, Chiu SS. 2021; Primary varicella zoster infection compared to varicella vaccine reactivation associated meningitis in immunocompetent children. J Paediatr Child Health. 57:19–25. DOI: 10.1111/jpc.15303. PMID: 33295075.
14. Chan Y, Smith D, Sadlon T, Scott JX, Goldwater PN. 2007; Herpes zoster due to Oka vaccine strain of varicella zoster virus in an immunosuppressed child post cord blood transplant. J Paediatr Child Health. 43:713–5. DOI: 10.1111/j.1440-1754.2007.01191.x. PMID: 17854459.
Fig. 1
Changes in aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total bilirubin levels during hospitalization. Acyclovir was administered on the fourth day of hospitalization and intravenous immunoglobulin was administered on the fifth day of hospitalization. After the sixth day of hospitalization, AST and ALT levels began to decrease. Hepatitis A virus immunoglobulin G antibody and hepatitis A virus immunoglobulin M antibody were examined by radioimmunoassay. Hepatitis B surface antigen was examined by chemiluminescent microparticle immunoassay. Anti-hepatitis C virus and varicella zoster virus immunoglobulin M antibody were examined by enzyme-linked immunosorbent assay. Cytomegalovirus antigen was examined by monoclonal antibody stain. Polyomavirus examined by real-time polymerase chain reaction. Varicella zoster virus immunoglobulin G antibody was examined by chemiluminescent immunoassay.
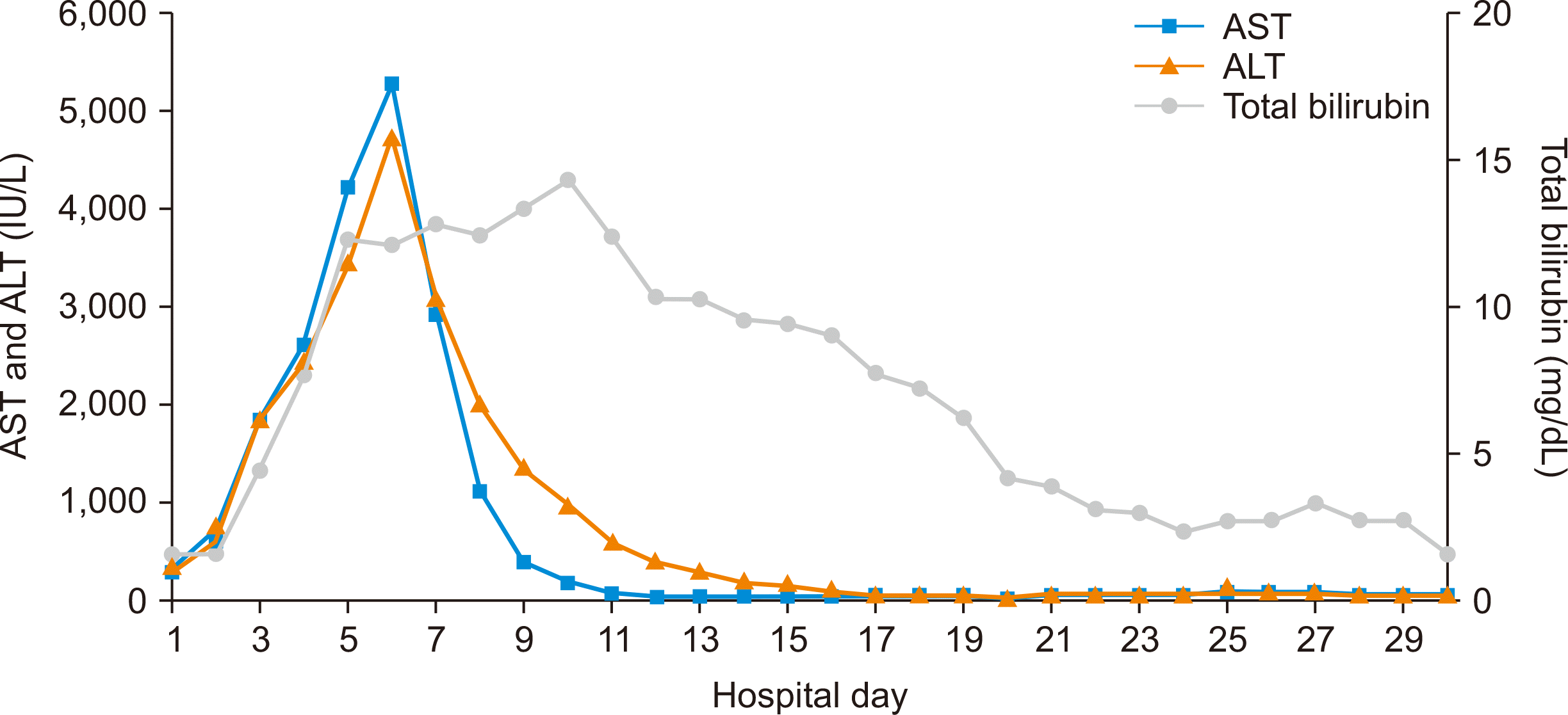
Table 1
Molecular and microbiological data during hospitalization




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download