Abstract
Background
Increasingly many patients are being relisted for repeat kidney transplantation due to longer survival times after transplantation. This study compared the outcomes of second living donor kidney transplantations (LDKTs) with those of first LDKTs.
Methods
Data were collected retrospectively for 1,429 LDKTs performed from 1995 to 2020 at Samsung Medical Center. The demographics of recipients and donors, immunologic factors, and outcomes of second LDKTs were compared to those of first LDKTs.
Results
Among 1,429 cases of LDKT, 1,355 were first LDKT cases and 74 were second LDKT cases. Basic demographic data were comparable for the two groups of patients. The 5- and 10-year graft survival rates were 94% and 84% for first LDKTs and 96% and 86% for second LDKTs, respectively, with neither difference statistically significant (P=0.399). The 5- and 10-year patient survival rates were 98% and 94% for the first and 96% and 93% for the second LDKTs, respectively; neither difference was statistically significant (P=0.766). Multivariate analysis confirmed that a history of previous transplantation was not a statistically significant risk factor for graft loss (hazard ratio [HR], 0.83; P=0.677) or patient death (HR, 1.68; P=0.396).
Since the introduction of kidney transplantation in South Korea in 1969, kidney transplantation rates have been increasing markedly. According to the Korean Network for Organ Sharing, 554, 1,289, and 2,293 cases were performed in 2000, 2010, and 2019, respectively—a doubling every 10 years. Over the last two decades, more than 70% of the patients receiving kidney transplantation were between 20 to 59 years old. As life expectancy after transplantation has increased, most of these cases will likely be candidates for repeat kidney transplantation at some point in their lifetime [1] due to the limited lifespan of allografts (10 years) [2,3].
The number of patients rejoining the kidney waitlist due to allograft loss is increasing, accounting for 9.2% of total transplant patients according to Korean national data. Among 26,074 patients on the waitlist for kidney transplantation, 2,399 would be undergoing repeat surgery. This phenomenon is occurring not only in Korea, but also worldwide, especially in the United States [4,5]. This study, therefore, sought to understand the outcomes of second kidney transplantation as the need for repeat transplantation is increasing.
This study sought to clarify the efficacy and safety of repeat renal transplantation compared to those of first transplants. Only cases of living donor kidney transplantation (LDKT) were included to reduce heterogeneity in donor-related factors. This study had the largest number of patients in a study on this topic to date, and could therefore provide stronger evidence than previous studies.
This study was reviewed and approved by the Institutional Review Board (IRB) of Samsung Medical Center (IRB No. 2020-03-214-002). The requirement for informed consent was waived by the IRB due to the retrosepctive nature of this study.
Patients from a single institution (Samsung Medical Center, Seoul, Korea) who received treatment from February 1995 to May 2020 were analyzed. Only adult patients who underwent LDKT were included. Cases with deceased donors, three or more previous renal transplants, or multiple organ transplants were excluded.
Demographics (age, sex, and body mass index [BMI]), underlying kidney disease, diabetes mellitus (DM), and hypertension for both recipients and donors were collected. Immunologic information such as panel-reactive antibody (PRA) identification, human leukocyte antigen (HLA) profile, donor-specific antibody (DSA) information, and the serum creatinine (sCr) level were reviewed. Surgical technique (total operative time, cold ischemic time, anastomosis time) and in-hospital postoperative complications were also recorded.
The primary study outcomes were death-censored graft survival and patient survival for first and second LDKT grafts. The secondary outcomes were changes in graft function over time, postoperative complications, and risk factors associated with graft failure and patient mortality. Recipients of ABO-incompatible transplants required a preconditioning process. One month prior to surgery, patients received rituximab injection, and plasmapheresis was performed to lower the immunoglobulin G titer under 1:32. If the titer was 1:256 or higher, intravenous immunoglobulin was also administered. Antithymocyte globulin (ATG) was used as an inductive agent.
Continuous variables were described as mean and standard deviation and analyzed with the independent t-test or the Mann-Whitney U-test. Categorical data were described as number and percentage and analyzed with the chi-square or Fisher’s exact test. Generalized estimating equations were used to compare sCr over time. Death-censored graft survival was measured using a graph generated by the Kaplan-Meier method. The prognosis was compared using a Cox proportional-hazards model and described as the hazard ratio (HR) and 95% confidence interval (CI). For multivariable analysis, variables with P<0.05 in the univariable analysis were selected. Statistical significance was defined as P<0.05. All statistical analyses used SAS ver. 9.4 (SAS Institute, Cary, NC, USA) and R 4.0.0 (R Studio, Vienna, Austria).
A total of 1,429 patients met the inclusion criteria. Of them, 1,355 received a first LDKT and 74 received a second LDKT. There were no statistically significant differences in sex, age, prevalence of DM, or hypertension between the two groups. The BMI of recipients of first transplants, however, was significantly higher than that of recipients of second transplants (P=0.001). There were also significant differences in the causes of renal failure between the two groups. The demographic characteristics of donors between the two groups did not show statistically significant differences.
There were significant differences in immunologic risk, as shown by a DSA (+) rate of 24% in the second LDKT patients versus 6.9% in the first LDKT patients (P<0.001). Inductive immunosuppressive agent use also showed significant differences (P<0.001), as 81% of second LDKT patients were treated with ATG, versus the first KT patients, of whom 32% received no inductive agent, 40% basiliximab, and 29% ATG. There were no statistically significant differences in the total operative time, cold ischemic time, or anastomosis time between the two groups (Table 1).
The 5-year graft survival rate was 94% in the first LDKT group and 96% in the second LDKT group. The 10-year graft survival rate was 84% in the first LDKT group and 86% in the second LDKT group. These differences were not statistically significant (P=0.399) (Fig. 1). During 20 years of follow-up, 199 graft losses were observed in the first LDKT group and 5 graft losses in the second LDKT group.
The 5-year patient survival rate was 98% in the first LDKT group and 96% in the second LDKT group. The 10-year patient survival rate was 94% in the first LDKT group and 93% in the second LDKT group. These differences were not statistically significant (P=0.766) (Fig. 2). Among the 64 deaths in the first LDKT group, 44 had functioning grafts, while all three patients who died in the second LDKT group had functioning grafts.
Univariate analysis showed that a history of kidney transplantation, DM (recipient), BMI (recipient), age (donor), ABO-incompatible transplantation, and the number of HLA II mismatches, were associated with an increased risk of graft failure. Subsequent multivariate analysis confirmed that the age of the donor (HR, 1.03; P<0.001) and the number of HLA II mismatches (HR, 1.63; P=0.006) increased the risk of graft failure of LDKT (Table 2). Univariate analysis showed that a history of kidney transplantation, age (recipient), DM (recipient), age (donor), hypertension (donor), and number of HLA II mismatches were associated with patients’ overall survival. Multivariate analysis showed that age (recipient: HR, 1.07; P<0.001), hypertension (donor: HR, 2.51; P=0.046), and number of HLA II mismatches (HR, 1.97; P=0.016) were associated with higher risk of mortality (Table 3). A history of previous transplantation, however, was not a statistically significant risk factor for graft or patient survival.
This study analyzed sCr for 10 years after transplantation to evaluate graft function over time. Results showed the average sCr of both groups increased over time, though there was no statistically significant difference in the rate of change between the groups (P=0.238). In short, there were no significant differences in graft function over time between the recipients of first and second LDKTs.
Postoperative bleeding—defined as requiring transfusion after surgery—was the most common postoperative complication in both groups (13% vs. 12% in the first and second KT groups, respectively), followed by lymphocele, wound complication, and ureteral leakage. There was no significant difference in frequency or type of complication between the two groups (P=0.340).
Medical complications such as infections and malignancy showed no statistically significant differences between the two groups. The prevalence of cytomegalovirus antigenemia was 53.4% (727/1,378) versus 48.6% (36/74) in first and second LDKTs, respectively (P=0.47), while that of BK virus was 15.7% (217/1,378) versus 13.5% (10/74) for first and second LDKTs, respectively (P=0.74). The incidence of malignancy after transplantation was 6.1% (84/1378) versus 4.1% (3/74) for first and second LDKTs, respectively (P=0.62).
Among the 74 patients in the repeat transplantation cohort, of whom one out of six received no inductive therapy (17%), one of eight received basiliximab (13%), and two of 60 received ATG (3.4%) experienced graft failure within 10 years of follow-up. While these numbers were not statistically significant—due in part to the small sample size—ATG may have had positive impacts on graft survival compared to no agent or basiliximab (Table 4).
This study analyzed the clinical data of 1,429 patients who underwent LDKT. Graft and patient survival rates were not significantly different between the first and second transplant groups (P=0.399 and P=0.766, respectively). Graft function (as measured by sCr) over time was comparable (P=0.238). Multivariate analysis, furthermore, showed that repeat transplantation increased neither the risk of graft failure (HR, 0.83; P=0.677) nor patient death (HR, 1.68; P=0.396). These results support the hypothesis that second renal transplantation with a living donor kidney is as safe and effective as first transplantation procedures, which is consistent with previous studies of repeat renal transplantation. Pour-Reza-Gholi et al. [6] compared the clinical outcomes of 103 cases of second renal transplantation with 2,009 cases of first transplants and showed comparable 5-year patient survival. As that study included both living and deceased donors, however, there were limitations involving the heterogeneity of donor-related factors. El-Agroudy et al. [7] also compared the outcomes of 1,352 first transplants and 52 cases of second renal transplants from living related donors only and showed no significant differences in overall patient survival and graft survival between the two groups. As that study included Egyptian patients only, however, there could be differences in demographic characteristics [7].
Patients who consider repeat transplantation after graft loss have two options: waiting for a deceased donor or looking for an appropriate living donor. Previous studies have shown that repeat transplantation has clear survival benefits compared to remaining on dialysis. Ojo et al. [8] analyzed graft failure in 19,208 patients and found that retransplantation (risk ratio [RR], 0.77; P<0.01) reduced the risk of long-term patient mortality compared to those who remained on the waitlist (RR=1.0). Rao et al. [9] also showed that retransplantation was associated with a covariate-adjusted 50% reduction in mortality relative to remaining on dialysis after graft loss. Repeat kidney transplantation, however, can be challenging due to organ shortages [4]. The key advantage of LDKT is that it can reduce the wait time before transplantation compared to deceased donor transplantation [10]. With an appropriate living donor for repeat transplantation, therefore, the risk of mortality can be reduced compared to remaining on the waitlist. As repeat renal transplantation from living donor has comparable outcomes compared to first transplant, it is a reasonable choice for patients with allograft loss.
Recipients of repeat transplants did show higher immunologic risk than those of first transplants [11,12], with the presence of DSA significantly higher in patients with prior transplantation (6.9% vs. 24%, P<0.001) (Table 1). Despite these immunologic disadvantages, our results showed comparable graft survival between first and second transplants. The only statistically significant difference in immunosuppressive strategy between the two groups was the use of an inductive agent (P<0.001). More than 80% of patients who received repeat transplantation were treated with ATG, while 40% of patients who underwent first kidney transplantation used basiliximab (a monoclonal antibody against CD25, an IL-2 receptor alpha chain) or ATG (11%).
ATG blocks T cell membrane proteins globally, depleting antibodies and producing profound and durable lymphopenia, while basiliximab specifically blocks the IL-2 signal pathway [13]. This study compared patient outcomes, including immunologic vulnerability, to determine whether the global immunosuppressive effects of ATG over basiliximab had a significant impact. Table 4 showed that the use of ATG seems to have protective effects on graft survival compared to no agent or the use of basiliximab. Univariate analysis, however, found no increased risk of graft failure by the type of inductive agent (Table 2). Previous studies do not indicate ATG as being superior to no agent or basiliximab as an inductive agent for repeat transplantation for graft and patient survival [14-16]. We are therefore cautious in concluding that use of ATG in repeat transplantation truly contributed to the improved results, even with the immunologic disadvantages in repeat transplantation patients in our study. Further investigation of inductive agents in repeat transplantation is needed.
This study has some limitations, including its retrospective design and the analysis of data from a single center. Repeat transplantation patients are also subject to selection bias, as they tend to be healthier or show better kidney performance than those who return to dialysis after allograft loss. The relatively small size of the repeat transplantation group was also a challenge for analysis. This study is nonetheless valuable, as it provides more robust evidence of the safety and efficacy of repeat kidney transplantation specifically with living donors. This study shows that repeat renal transplantation with living donor kidneys offers comparable graft survival, patient survival, and graft function to first transplantation procedures, with no significant increase in complications. Repeat kidney transplantation with living donors is therefore a reasonable choice to reduce the waiting time for transplantation.
ACKNOWLEDGMENTS
Conflict of Interest
Jong Man Kim is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflict of interest relevant to this article was reported.
Funding/Support
This study was supported by research grant from the Korean Society for Transplantation (2022-00-01001-008).
Author Contributions
Conceptualization: NO, MJK, KWL. Data curation: all authors. Formal analysis: NO, HC, SYW. Methodology: all authors. Project administration: NO, MJK, KWL. Visualization: NO, MJK, KWL. Writing–original draft: NO, MJK, KWL. Writing–review & editing: all authors.
REFERENCES
1. Tonelli M, Wiebe N, Knoll G, Bello A, Browne S, Jadhav D, et al. 2011; Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant. 11:2093–109. DOI: 10.1111/j.1600-6143.2011.03686.x. PMID: 21883901.
2. Lamb KE, Lodhi S, Meier-Kriesche HU. 2011; Long-term renal allograft survival in the United States: a critical reappraisal. Am J Transplant. 11:450–62. DOI: 10.1111/j.1600-6143.2010.03283.x. PMID: 20973913.
3. Poggio ED, Augustine JJ, Arrigain S, Brennan DC, Schold JD. 2021; Long-term kidney transplant graft survival: making progress when most needed. Am J Transplant. 21:2824–32. DOI: 10.1111/ajt.16463. PMID: 33346917.
4. Magee JC, Barr ML, Basadonna GP, Johnson MR, Mahadevan S, McBride MA, et al. 2007; Repeat organ transplantation in the United States, 1996-2005. Am J Transplant. 7(5 Pt 2):1424–33. DOI: 10.1111/j.1600-6143.2007.01786.x. PMID: 17428290.
5. Fiorentino M, Gallo P, Giliberti M, Colucci V, Schena A, Stallone G, et al. 2020; Management of patients with a failed kidney transplant: what should we do? Clin Kidney J. 14:98–106. DOI: 10.1093/ckj/sfaa094. PMID: 33564409. PMCID: PMC7857798.
6. Pour-Reza-Gholi F, Nafar M, Saeedinia A, Farrokhi F, Firouzan A, Simforoosh N, et al. 2005; Kidney retransplantation in comparison with first kidney transplantation. Transplant Proc. 37:2962–4. DOI: 10.1016/j.transproceed.2005.08.034. PMID: 16213274.
7. El-Agroudy AE, Wafa EW, Bakr MA, Donia AF, Ismail AM, Shokeir AA, et al. 2004; Living-donor kidney retransplantation: risk factors and outcome. BJU Int. 94:369–73. DOI: 10.1111/j.1464-410X.2004.04934.x. PMID: 15291869.
8. Ojo A, Wolfe RA, Agodoa LY, Held PJ, Port FK, Leavey SF, et al. 1998; Prognosis after primary renal transplant failure and the beneficial effects of repeat transplantation: multivariate analyses from the United States Renal Data System. Transplantation. 66:1651–9. DOI: 10.1097/00007890-199812270-00014. PMID: 9884254.
9. Rao PS, Schaubel DE, Wei G, Fenton SS. 2006; Evaluating the survival benefit of kidney retransplantation. Transplantation. 82:669–74. DOI: 10.1097/01.tp.0000235434.13327.11. PMID: 16969291.
10. Meier-Kriesche HU, Kaplan B. 2002; Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: a paired donor kidney analysis. Transplantation. 74:1377–81. DOI: 10.1097/00007890-200211270-00005. PMID: 12451234.
11. Lefaucheur C, Suberbielle-Boissel C, Hill GS, Nochy D, Andrade J, Antoine C, et al. 2008; Clinical relevance of preformed HLA donor-specific antibodies in kidney transplantation. Am J Transplant. 8:324–31. DOI: 10.1111/j.1600-6143.2007.02072.x. PMID: 18162086.
12. Amrouche L, Aubert O, Suberbielle C, Rabant M, Van Huyen JD, Martinez F, et al. 2017; Long-term outcomes of kidney transplantation in patients with high levels of preformed DSA: the Necker high-risk transplant program. Transplantation. 101:2440–8. DOI: 10.1097/TP.0000000000001650. PMID: 28114171.
13. Halloran PF. 2004; Immunosuppressive drugs for kidney transplantation. N Engl J Med. 351:2715–29. DOI: 10.1056/NEJMra033540. PMID: 15616206.
14. Mourad G, Garrigue V, Squifflet JP, Besse T, Berthoux F, Alamartine E, et al. 2001; Induction versus noninduction in renal transplant recipients with tacrolimus-based immunosuppression. Transplantation. 72:1050–5. DOI: 10.1097/00007890-200109270-00012. PMID: 11579299.
15. Brennan DC, Daller JA, Lake KD, Cibrik D. Del Castillo D; Thymoglobulin Induction Study Group. 2006; Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med. 355:1967–77. DOI: 10.1056/NEJMoa060068. PMID: 17093248.
16. Schold J, Poggio E, Goldfarb D, Kayler L, Flechner S. 2015; Clinical outcomes associated with induction regimens among retransplant kidney recipients in the United States. Transplantation. 99:1165–71. DOI: 10.1097/TP.0000000000000507. PMID: 25606788.
Fig. 1
Kaplan-Meier plot for death-censored graft survival for 10 years. The 5-year graft survival rates were 94% and 96%, and the 10-year rates were 84% and 86% in first and second living donor kidney transplantation (LDKT) patients, respectively. The differences were not statistically significant (P=0.399).
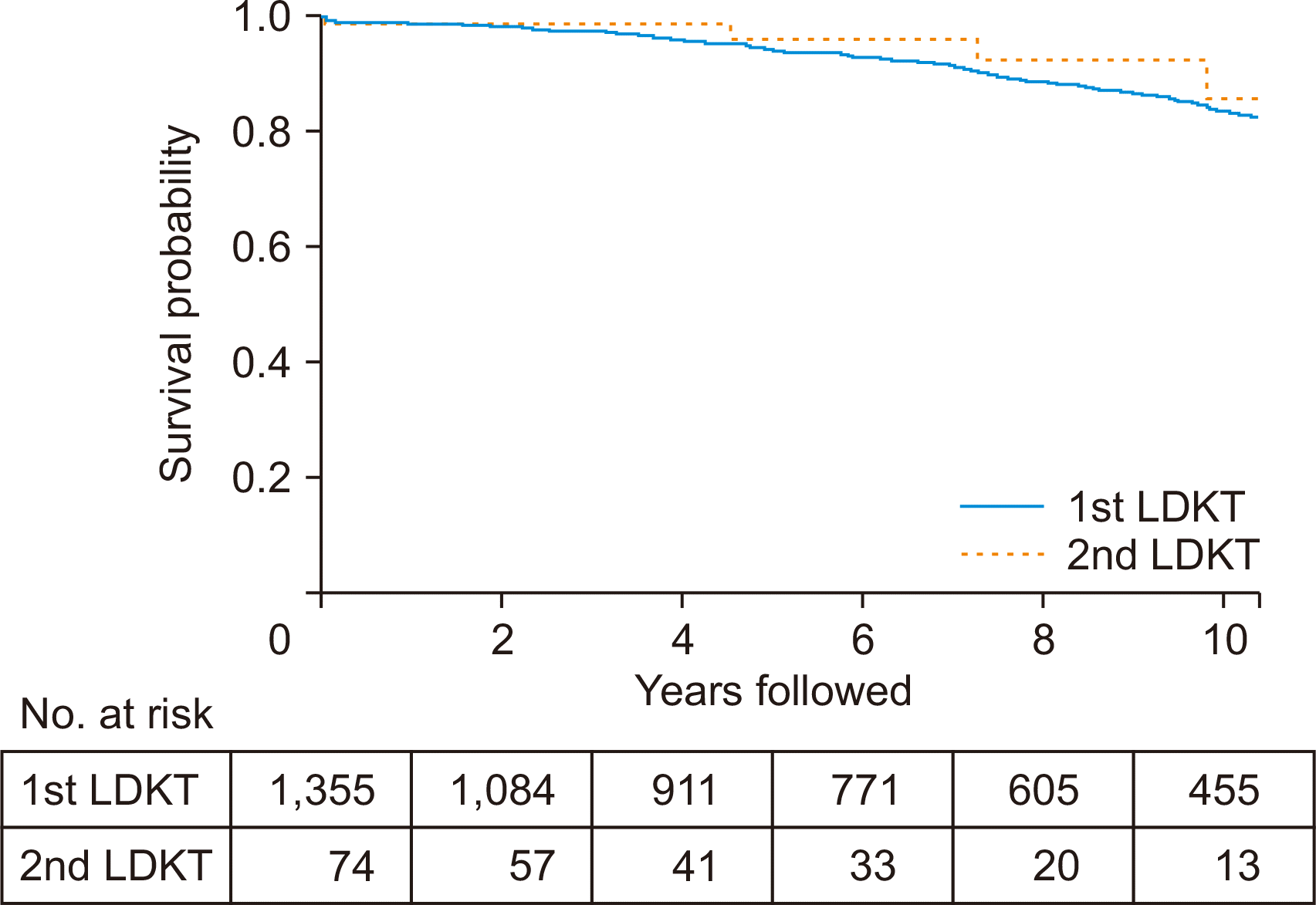
Fig. 2
Kaplan-Meier plot for patient survival for 10 years. The 5-year patient survival rates were 98% and 96%, and the 10-year rates were 94% and 93% in first and second living donor kidney transplantation (LDKT) patients, respectively. The differences were not statistically significant (P=0.766).
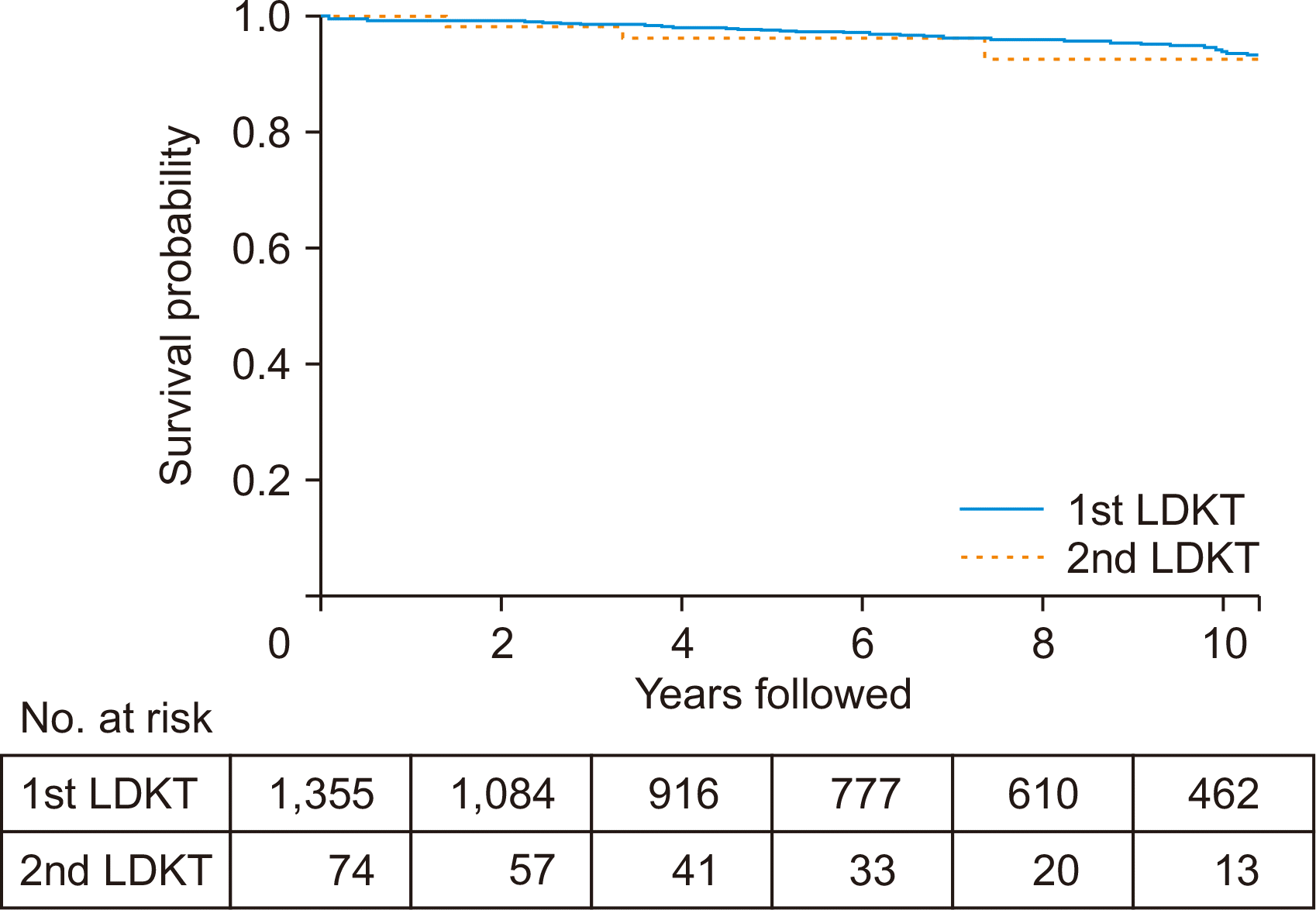
Table 1
Clinicopathologic characteristics by history of kidney transplantation
LDKT, living donor kidney transplantation; BMI, body mass index; DM, diabetes mellitus; HTN, hypertension; GN, glomerular nephropathy; ADPCKD, autosomal dominant polycystic kidney disease; sCr, serum creatinine; ATG, anti-thymocyte globulin; CNI, calcineurin inhibitor; mHLA, monocytic human leukocyte antigen; DSA, donor-specific antibody.
Table 2
Risk factors associated with graft failure
Table 3
Risk factors associated with patient death




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download