1. Strong RW, Lynch SV, Ong TH, Matsunami H, Koido Y, Balderson GA. 1990; Successful liver transplantation from a living donor to her son. N Engl J Med. 322:1505–7. DOI:
10.1056/NEJM199005243222106. PMID:
2336076.
3. Lo CM, Fan ST, Liu CL, Lo RJ, Lau GK, Wei WI, et al. 1997; Extending the limit on the size of adult recipient in living donor liver transplantation using extended right lobe graft. Transplantation. 63:1524–8. DOI:
10.1097/00007890-199705270-00027. PMID:
9175822.
4. Chan SC, Chan AC, Sharr WW, Chok KS, Cheung TT, Fan ST, et al. 2014; Perpetuating proficiency in donor right hepatectomy for living donor liver transplantation. Asian J Surg. 37:65–72. DOI:
10.1016/j.asjsur.2013.09.001. PMID:
24210956.
5. Nakamura T, Tanaka K, Kiuchi T, Kasahara M, Oike F, Ueda M, et al. 2002; Anatomical variations and surgical strategies in right lobe living donor liver transplantation: lessons from 120 cases. Transplantation. 73:1896–903. DOI:
10.1097/00007890-200206270-00008. PMID:
12131684.
6. Shoreem H, Gad EH, Soliman H, Hegazy O, Saleh S, Zakaria H, et al. 2017; Small for size syndrome difficult dilemma: lessons from 10 years single centre experience in living donor liver transplantation. World J Hepatol. 9:930–44. DOI:
10.4254/wjh.v9.i21.930. PMID:
28824744. PMCID:
PMC5545138.
7. Dahm F, Georgiev P, Clavien PA. 2005; Small-for-size syndrome after partial liver transplantation: definition, mechanisms of disease and clinical implications. Am J Transplant. 5:2605–10. DOI:
10.1111/j.1600-6143.2005.01081.x. PMID:
16212618.
8. Kiuchi T, Kasahara M, Uryuhara K, Inomata Y, Uemoto S, Asonuma K, et al. 1999; Impact of graft size mismatching on graft prognosis in liver transplantation from living donors. Transplantation. 67:321–7. DOI:
10.1097/00007890-199901270-00024. PMID:
10075602.
10. Ikegami T, Masuda Y, Ohno Y, Mita A, Kobayashi A, Urata K, et al. 2009; Prognosis of adult patients transplanted with liver grafts < 35% of their standard liver volume. Liver Transpl. 15:1622–30. DOI:
10.1002/lt.21716. PMID:
19877227.
11. Moon JI, Kwon CH, Joh JW, Jung GO, Choi GS, Park JB, et al. 2010; Safety of small-for-size grafts in adult-to-adult living donor liver transplantation using the right lobe. Liver Transpl. 16:864–9. DOI:
10.1002/lt.22094. PMID:
20583075.
12. Lee SD, Kim SH, Kim YK, Lee SA, Park SJ. 2014; Graft-to-recipient weight ratio lower to 0.7% is safe without portal pressure modulation in right-lobe living donor liver transplantation with favorable conditions. Hepatobiliary Pancreat Dis Int. 13:18–24. DOI:
10.1016/S1499-3872(14)60002-3.
13. Au KP, Chan SC, Chok KS, Chan AC, Wong TC, Sharr WW, et al. 2015; Durability of small-for-size living donor allografts. Liver Transpl. 21:1374–82. DOI:
10.1002/lt.24205. PMID:
26123155.
14. Ikegami T, Yoshizumi T, Sakata K, Uchiyama H, Harimoto N, Harada N, et al. 2016; Left lobe living donor liver transplantation in adults: what is the safety limit? Liver Transpl. 22:1666–75. DOI:
10.1002/lt.24611. PMID:
27540888.
15. Liu C, Song JL, Lu WS, Yang JY, Jiang L, Yan LN, et al. 2016; Hepatic arterial buffer response maintains the homeostasis of graft hemodynamics in patient receiving living donor liver transplantation. Dig Dis Sci. 61:464–73. DOI:
10.1007/s10620-015-3881-8. PMID:
26441282.
16. Wong TC, Fung JY, Cui TY, Sin SL, Ma KW, She BW, et al. 2021; The risk of going small: lowering GRWR and overcoming small-for-size syndrome in adult living donor liver transplantation. Ann Surg. 274:e1260–8. DOI:
10.1097/SLA.0000000000003824. PMID:
32209906.
17. Chan SC, Lo CM, Chok KS, Sharr WW, Cheung TT, Tsang SH, et al. 2011; Modulation of graft vascular inflow guided by flowmetry and manometry in liver transplantation. Hepatobiliary Pancreat Dis Int. 10:649–56. DOI:
10.1016/S1499-3872(11)60110-0.
18. Kelly DM, Demetris AJ, Fung JJ, Marcos A, Zhu Y, Subbotin V, et al. 2004; Porcine partial liver transplantation: a novel model of the "small-for-size" liver graft. Liver Transpl. 10:253–63. DOI:
10.1002/lt.20073. PMID:
14762864.
19. Nakamura S, Sakaguchi S, Hachiya T, Suzuki S, Nishiyama R, Konno H, et al. 1993; Significance of hepatic vein reconstruction in hepatectomy. Surgery. 114:59–64.
20. Lee SG, Park KM, Hwang S, Lee YJ, Kim KH, Ahn CS, et al. 2002; Adult-to-adult living donor liver transplantation at the Asan Medical Center, Korea. Asian J Surg. 25:277–84. DOI:
10.1016/S1015-9584(09)60192-5.
21. Pollard JJ, Nebesar RA. 1967; Altered hemodynamics in the Budd-Chiari syndrome demonstrated by selective hepatic and selective splenic angiography. Radiology. 89:236–43. DOI:
10.1148/89.2.236.
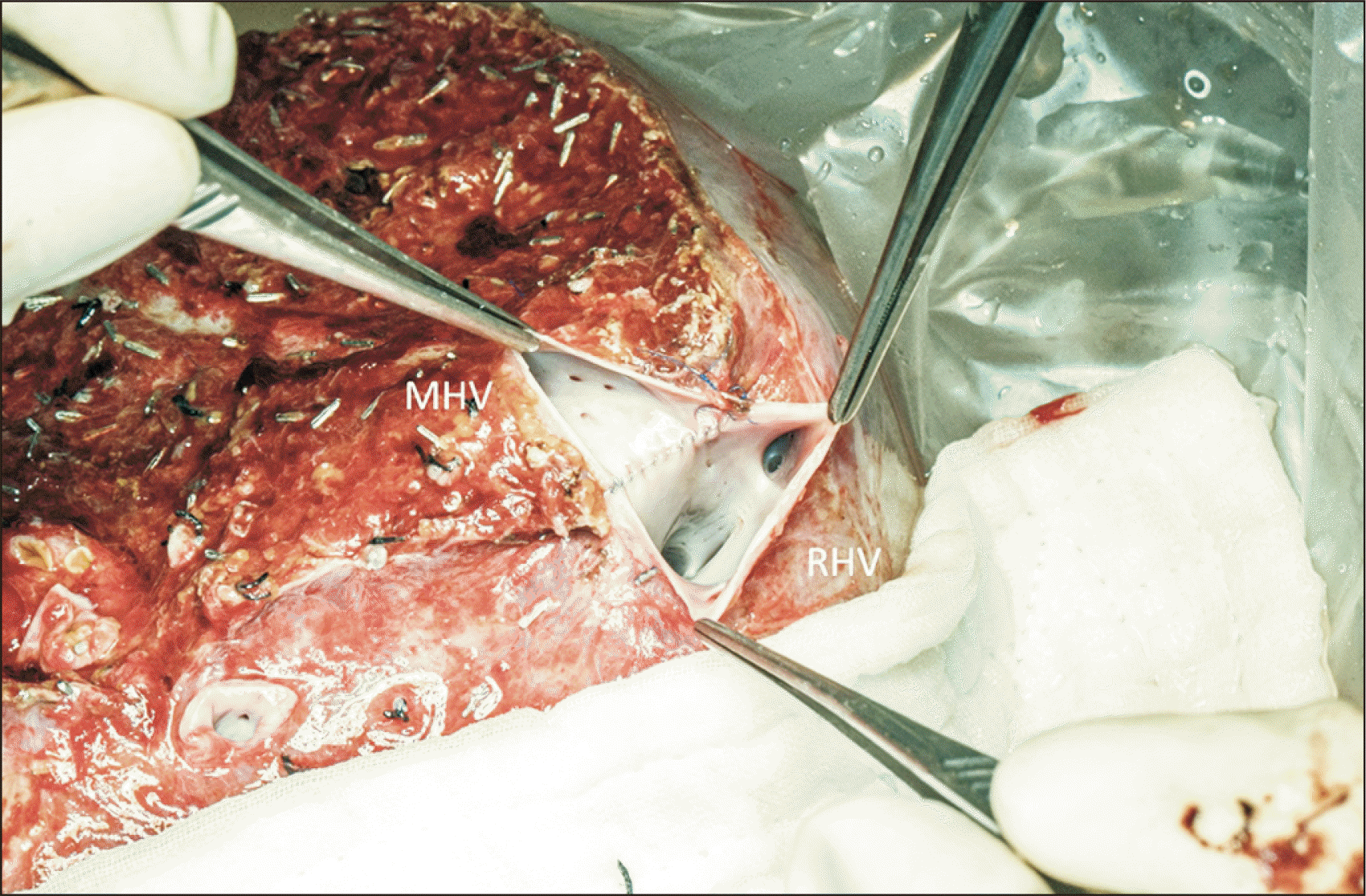
22. Liu CL, Zhao Y, Lo CM, Fan ST. 2003; Hepatic venoplasty in right lobe live donor liver transplantation. Liver Transpl. 9:1265–72. DOI:
10.1016/j.lts.2003.09.014. PMID:
14625826.
23. Nakamura S, Tsuzuki T. 1981; Surgical anatomy of the hepatic veins and the inferior vena cava. Surg Gynecol Obstet. 152:43–50.
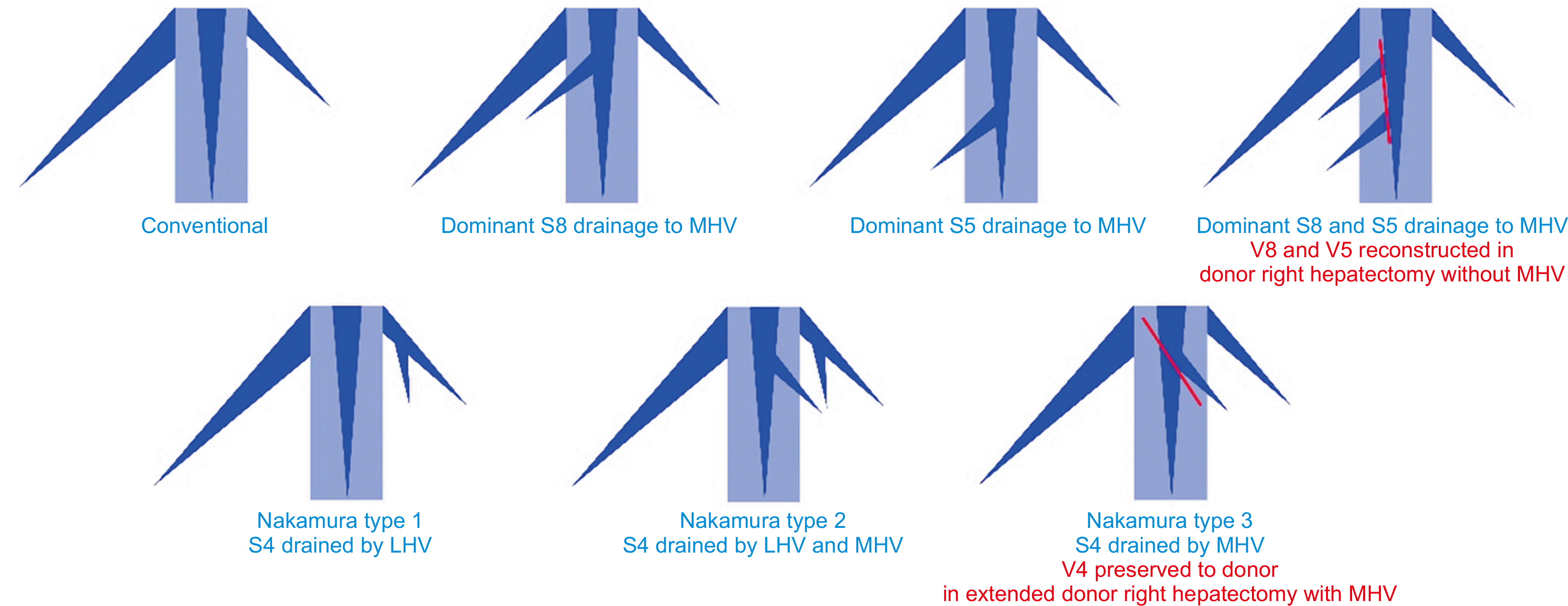
24. Chan SC, Lo CM, Liu CL, Wong Y, Fan ST, Wong J. 2004; Tailoring donor hepatectomy per segment 4 venous drainage in right lobe live donor liver transplantation. Liver Transpl. 10:755–62. DOI:
10.1002/lt.20114. PMID:
15162470.
25. Del Guercio LR, Cohn JD, Kazarian KK, Kinkhabwalla M. 1978; A shunt equation for estimating the splenic component of portal hypertension. Am J Surg. 135:70–5. DOI:
10.1016/0002-9610(78)90012-0.
26. Luca A, Miraglia R, Caruso S, Milazzo M, Gidelli B, Bosch J. 2006; Effects of splenic artery occlusion on portal pressure in patients with cirrhosis and portal hypertension. Liver Transpl. 12:1237–43. DOI:
10.1002/lt.20762. PMID:
16741929.
28. Boillot O, Delafosse B, Méchet I, Boucaud C, Pouyet M. 2002; Small-for-size partial liver graft in an adult recipient; a new transplant technique. Lancet. 359:406–7. DOI:
10.1016/S0140-6736(02)07593-1.
29. Iida T, Yagi S, Taniguchi K, Hori T, Uemoto S. 2007; Improvement of morphological changes after 70% hepatectomy with portocaval shunt: preclinical study in porcine model. J Surg Res. 143:238–46. DOI:
10.1016/j.jss.2006.11.020. PMID:
18023647.
30. Chan SC, Lo CM, Ng KK, Fan ST. 2010; Alleviating the burden of small-for-size graft in right liver living donor liver transplantation through accumulation of experience. Am J Transplant. 10:859–67. DOI:
10.1111/j.1600-6143.2010.03017.x. PMID:
20148811.
31. Yagi S, Uemoto S. 2012; Small-for-size syndrome in living donor liver transplantation. Hepatobiliary Pancreat Dis Int. 11:570–6. DOI:
10.1016/S1499-3872(12)60227-6.
32. Lei JY, Wang WT, Yan LN. 2012; Risk factors of SFSS in adult-to-adult living donor liver transplantation using the right liver: a single-center analysis of 217 cases. Hepatogastroenterology. 59:1491–7. DOI:
10.5754/hge11634.
33. Ma KW, Wong KH, Chan AC, Cheung TT, Dai WC, Fung JY, et al. 2019; Impact of small-for-size liver grafts on medium-term and long-term graft survival in living donor liver transplantation: a meta-analysis. World J Gastroenterol. 25:5559–68. DOI:
10.3748/wjg.v25.i36.5559. PMID:
31576100. PMCID:
PMC6767984.
34. Sureka B, Patidar Y, Bansal K, Rajesh S, Agrawal N, Arora A. 2015; Portal vein variations in 1000 patients: surgical and radiological importance. Br J Radiol. 88:20150326. DOI:
10.1259/bjr.20150326. PMID:
26283261. PMCID:
PMC4743455.
35. Schmidt S, Demartines N, Soler L, Schnyder P, Denys A. 2008; Portal vein normal anatomy and variants: implication for liver surgery and portal vein embolization. Semin Intervent Radiol. 25:86–91. DOI:
10.1055/s-2008-1076688. PMID:
21326549. PMCID:
PMC3036482.
36. Covey AM, Brody LA, Getrajdman GI, Sofocleous CT, Brown KT. 2004; Incidence, patterns, and clinical relevance of variant portal vein anatomy. AJR Am J Roentgenol. 183:1055–64. DOI:
10.2214/ajr.183.4.1831055. PMID:
15385304.
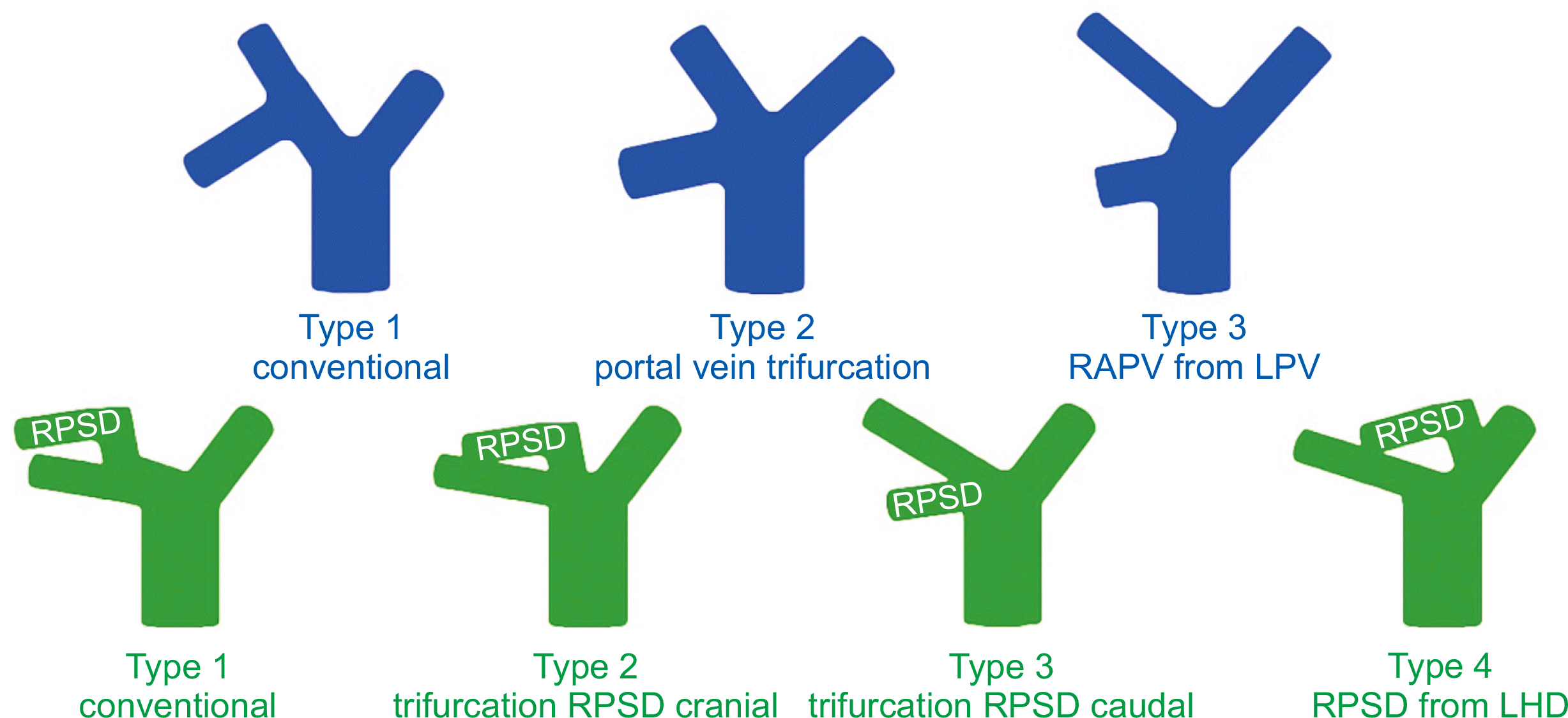
37. Thayer WP, Claridge JA, Pelletier SJ, Oh CK, Sanfey HA, Sawyer RG, et al. 2002; Portal vein reconstruction in right lobe living-donor liver transplantation. J Am Coll Surg. 194:96–8. DOI:
10.1016/S1072-7515(01)01085-7.
38. Lee SG, Hwang S, Kim KH, Ahn CS, Park KM, Lee YJ, et al. 2003; Approach to anatomic variations of the graft portal vein in right lobe living-donor liver transplantation. Transplantation. 75(3 Suppl):S28–32. DOI:
10.1097/01.TP.0000047028.97031.66. PMID:
12589136.
39. Yoshizumi T, Ikegami T, Kimura K, Uchiyama H, Ikeda T, Shirabe K, et al. 2014; Selection of a right posterior sector graft for living donor liver transplantation. Liver Transpl. 20:1089–96. DOI:
10.1002/lt.23924. PMID:
24890095.
40. Hori T, Kirino I, Uemoto S. 2015; Right posterior segment graft in living donor liver transplantation. Hepatol Res. 45:1076–82. DOI:
10.1111/hepr.12469. PMID:
25559984.
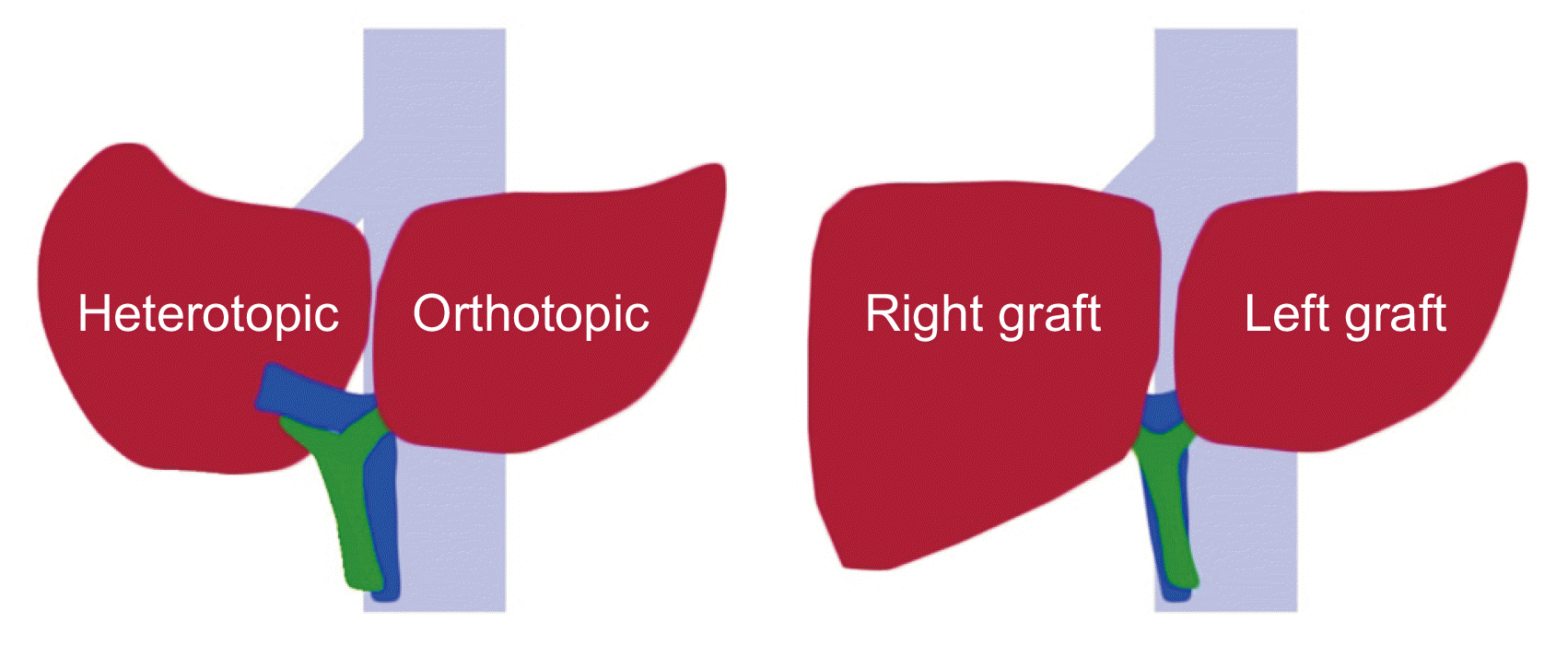
41. Lee S, Hwang S, Park K, Lee Y, Choi D, Ahn C, et al. 2001; An adult-to-adult living donor liver transplant using dual left lobe grafts. Surgery. 129:647–50. DOI:
10.1067/msy.2001.114218. PMID:
11331460.
42. Lee SG, Moon DB. Oniscu GC, Forsythe JL, Pomfret EA, editors. 2019. Dual-graft liver transplantation. Transplantation surgery. Springer;Berlin, Germany: p. 331–52. DOI:
10.1007/978-3-540-73796-4_14.
43. Xu Y, Chen H, Yeh H, Wang H, Leng J, Dong J. 2015; Living donor liver transplantation using dual grafts: Experience and lessons learned from cases worldwide. Liver Transpl. 21:1438–48. DOI:
10.1002/lt.24315. PMID:
26336078.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download