Abstract
Coronary artery spasm after heart transplantation is a very rare complication. In one observational study and many anecdotal reports, most cases of coronary artery spasm occurred more than 1 year after surgery and had good outcomes. However, cases of intractable coronary artery spasm during the early postoperative period resulting in fatality are limited. This report presents a case of two cardiac arrests caused by coronary artery spasms within a short period of time after heart transplantation.
Coronary artery spasm (CAS) is an extremely rare complication that can occur after cardiac transplantation [1-4]. In the largest single-center observational study on CAS in heart transplant recipients, 12 out of 247 patients (4.9%) experienced CAS, all within 1–3 years after heart transplantation [1]. Only a few reports on cases of CAS in the early posttransplant period have been published. Here, we report, for the first time, a case of two episodes of cardiac arrest due to severe CAS at very short intervals in the early postoperative period after heart transplantation.
This study was approved by the Institutional Review Board of Seoul National University Hospital (IRB No. H-2008-172-1152). The legal guardian of the patient provided written informed consent for publication of clinical details and images.
A 66-year-old man diagnosed with cardiac amyloidosis, who had received autologous stem cell transplantation 3 months prior to presentation, was admitted to the emergency department on account of acute decompensated heart failure. He was diagnosed with multiple myeloma, biopsy-proven renal and cardiac amyloidosis, and a reduced ejection fraction of 35%. After two cycles of bortezomib, thalidomide, and dexamethasone, autologous stem cell transplantation was performed, and a favorable response was achieved. After autologous stem cell transplantation, no recurrence of multiple myeloma was observed. Despite guideline-directed medical therapy, the symptoms of heart failure worsened in this patient. He presented to the emergency department with severe dyspnea (New York Heart Association class IV) and anuria. The patient was transferred to the intensive care unit and underwent conservative management, including oxygen supplementation via a nasal cannula, maintenance of blood pressure using vasoactive drugs, and volume reduction with continuous renal replacement therapy. During intensive care for 10 days, the patient’s general condition improved slightly; however, adequate urine output was not maintained, and we therefore continued renal replacement therapy. On the 10th day of hospitalization, the patient underwent orthotopic heart transplantation.
The donor was a previously healthy 39-year-old man diagnosed with brain death after a subarachnoid hemorrhage caused by a road traffic accident. The donor’s pretransplant echocardiography revealed a borderline left ventricular ejection fraction (50%) with no regional wall motion abnormality. There were no abnormal findings on inspection and palpation of the epicardial coronary vessels during donor heart procurement. The operation was performed without complications, and postoperative transesophageal echocardiography revealed normal biventricular contractility. A postoperative electrocardiogram illustrated normal sinus rhythm with nonsignificant ST-T abnormalities (Fig. 1A). After surgery, the recipient received supportive care, including immunosuppressants, inotropic agents, antibiotics, and continuous renal replacement therapy for oliguria in the intensive care unit.
In the morning of postoperative day 20, the patient experienced a cardiac arrest following sudden bradycardia and hypotension, during which three cycles of cardiopulmonary resuscitation were performed, and spontaneous circulation was restored. After the restoration of circulation, the patient’s vital signs stabilized (blood pressure, 142/66 mmHg; heart rate, 74 beats/min), but an electrocardiogram revealed tombstone signs in the inferior leads (Fig. 1B), strongly suggesting inferior wall myocardial infarction.
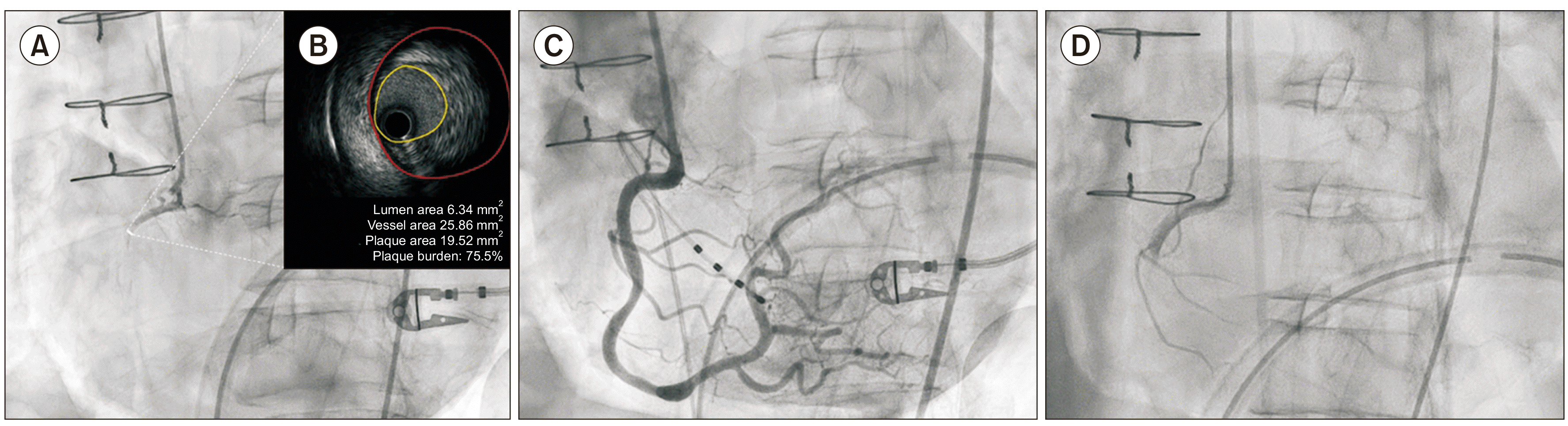
Invasive coronary angiography performed immediately after resuscitation revealed a normal left coronary artery but proximal total occlusion with multiple vasospastic lesions in the right coronary artery (RCA) (Fig. 2A). No thrombus was identified in the RCA. The occlusion was not responsive to multiple administrations of intracoronary nitrate, but eventually reversed after subsequent plain balloon angioplasty. Next, intravascular ultrasound (IVUS) was performed, and an eccentric large plaque burden (up to 75.5%) in the proximal RCA was observed (Fig. 2B). A drug-eluting stent (Sierra 4.0×18 mm) was implanted to treat the plaque and prevent a fatal focal spasm in the RCA (Fig. 2C). A follow-up electrocardiogram revealed resolved ischemic changes (Fig. 1C); echocardiography revealed preserved left ventricular systolic function (ejection fraction, 54%), with no regional wall motion abnormality. Endomyocardial biopsy was not performed after cardiac arrest. After the procedure, intravascular nicorandil was continuously infused (1 mg/hr), while other vasodilators were not prescribed because of the pre-existing hypotension (94/54 mmHg with the support of vasoactive drugs: norepinephrine (16 μg/min), dopamine (10 μg/kg/min), and dobutamine (2.5 μg/kg/min). For antiplatelet therapy, clopidogrel (37.5 mg daily) was administered owing to severe thrombocytopenia (lower than 10,000/mL without transfusion).
Four days after the first cardiac arrest, a second episode occurred unexpectedly with a rapid drop in blood pressure and heart rate again. Spontaneous circulation returned after 2 minutes of cardiopulmonary resuscitation, immediately after which his blood pressure was 90/48 mmHg and his heart rate 64 beats/min. An electrocardiogram revealed a wide QRS interval with ST-segment elevation in the inferior leads (Fig. 1D). Coronary angiography demonstrated a normal left coronary artery, but a severe diffuse RCA spasm occurred despite multiple intracoronary nitrate and nicorandil injections, sparing only the stent-implanted proximal lesion (Fig. 2D). The spasm gradually worsened and was accompanied by hypotension, bradycardia, and atrioventricular dissociation during the procedure. A temporary pacemaker and an intra-aortic balloon pump were inserted, and venoarterial extracorporeal membrane oxygenation was administered. Despite the maximum supportive therapy, the patient died 8 hours after the second cardiac arrest episode.
Although extremely rare, a few cases have been published describing recipient hearts that exhibited vasospastic responses early after cardiac transplantation [2-4]. In two of these cases, RCA spasms repeatedly occurred within 4 weeks of cardiac transplantation, but the patients were stabilized using a calcium channel blocker and discharged without recurrence. One case report described a patient who survived with venoarterial extracorporeal membrane oxygenation and intermittent nitroglycerin infusion for severe and diffuse vasospasm in both the left and right coronary arteries during cardiac transplantation. In all previous cases, CAS was immediately reversed after intracoronary nitrate infusion. Therefore, this is the first case of intractable CAS that did not respond to intracoronary nitrate or vasodilating agents occurring early after cardiac transplantation.
This case emphasizes several lessons. First, recurrent and intractable vasospasm, necessitating mechanical circulatory support (MCS), can occur during the early phase of heart transplantation. The proposed mechanisms of CAS include involvement of the autonomic nervous system, inflammation, endothelial dysfunction, smooth muscle cell hypercontractility, oxidative stress, and genetic factors [5]. Notwithstanding autonomic denervation in the transplanted heart, abnormal vascular reactivity, endothelial dysfunction, or microvascular dysfunction might be involved in the pathogenesis of CAS during heart transplant. Atherosclerosis of the donor heart can also account for vasoreactivity [2]. The sufficient use of vasodilating agents, such as calcium channel blockers and nitrates, is important in patients at high risk for CAS [6]. Early initiation of MCS is another option if optimal medical treatment is not possible [4]. In this case, only low-dose intravenous nicorandil was administered because of the patient’s low blood pressure after surgery, and MCS was eventually applied after the second CAS.
Second, stent implantation for coronary spasm should be carefully determined in clinical practice. A few days after the first cardiac arrest due to the RCA spasm unresponsive to multiple vasodilator administration, which was corrected by balloon angioplasty and stent implantation, the patient experienced a second cardiac arrest, leading to death. Although some recently published case reports have described medically unresponsive coronary spasms that improved after stent implantation [7,8], percutaneous coronary intervention is not generally recommended for coronary spasms because of the possibility of multivessel spasm or stent thrombosis after the procedure [9]. In the current case, the lesion may not have been related to myocardial ischemia, given the lumen area confirmed by IVUS. Stent implantation at the non-ischemia-causing spastic coronary artery may not be the treatment of choice, even after considering the critical state of the patient.
Third, a pretransplant coronary angiogram might be helpful to confirm atherosclerotic lesions of the donor heart and detect the presence of vasoreactivity in the recipient heart. A consensus statement for donor heart procurement recommends avoiding donor-transmitted coronary artery disease as it may result in up to three-fold increased risk of primary graft dysfunction [10]. Although the donor in this case was not indicated to undergo left heart catheterization before transplantation according to the consensus statement, and no abnormalities were found during the intraoperative inspection and palpation of the coronary arteries, a high plaque burden of the RCA was found on intracoronary imaging. Although routinely performing pretransplant coronary evaluations may be impractical, the benefits and risks should be considered in high-risk patients to predict early postoperative coronary vasospasm.
Intractable CAS early after a heart transplant is highly uncommon, but nonetheless represents a possible cause of cardiac arrest leading to death. Donor heart coronary evaluation before transplantation and appropriate use of vasodilators during the perioperative period might be helpful for reducing the incidence of early CAS.
ACKNOWLEDGMENTS
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: JC, JK. Data curation: JK, SHS, HYH. Formal analysis: JC, JK. Methodology: SHS, HYH. Project administration: JK, HJC. Visualization: JC, HYL. Writing–original draft: JC, JK. Writing–review & editing: JK, HYL, HYH, HJC.
REFERENCES
1. Boffa GM, Livi U, Grassi G, Casarotto D, Isabella G, Cardaioli P, et al. 2000; Angiographic presentation of coronary artery spasm in heart transplant recipients. Int J Cardiol. 73:67–74. DOI: 10.1016/S0167-5273(99)00225-9. PMID: 10748313.
2. Pagnoni M, Regamey J, Adjedj J, Rogati G, Muller O, Tozzi P. 2019; Case report - coronary vasospasm in transplanted heart: a puzzling phenomenon. BMC Cardiovasc Disord. 19:305. DOI: 10.1186/s12872-019-01280-8. PMID: 31856732. PMCID: PMC6924038.
3. Pistono M, Brentana L, Gnemmi M, Imparato A, Temporelli PL, Zingarelli E, et al. 2009; Early right coronary vasospasm presenting with malignant arrhythmias in a heart transplantation recipient without allograft vasculopathy. Int J Cardiol. 131:e120–3. DOI: 10.1016/j.ijcard.2007.07.078. PMID: 17950482.
4. Sheu R, Berfield K, Jones S, Pal J, Mackensen GB. 2017; Intraoperative acute multivessel coronary vasospasm in cardiac allograft: a case report. A A Case Rep. 9:328–31. DOI: 10.1213/XAA.0000000000000608. PMID: 28727596.
5. Hung MJ, Hu P, Hung MY. 2014; Coronary artery spasm: review and update. Int J Med Sci. 11:1161–71. DOI: 10.7150/ijms.9623. PMID: 25249785. PMCID: PMC4166862.
6. Takagi Y, Takahashi J, Yasuda S, Miyata S, Tsunoda R, Ogata Y, et al. 2013; Prognostic stratification of patients with vasospastic angina: a comprehensive clinical risk score developed by the Japanese Coronary Spasm Association. J Am Coll Cardiol. 62:1144–53. DOI: 10.1016/j.jacc.2013.07.018. PMID: 23916938.
7. Cho S, Kang TS. 2018; Multi-vessel intractable coronary spasm development in a patient with aborted sudden cardiac death: a case study with intravascular ultrasound findings. Yeungnam Univ J Med. 35:121–6. DOI: 10.12701/yujm.2018.35.1.121. PMID: 31620582. PMCID: PMC6784666.
8. Jiang M, Kaplan RM, Peigh G, Cheema B, Sawicki KT, Pfenniger A, et al. 2020; Vasospastic arrest: a heart-stopping case of prinzmetal angina. JACC Case Rep. 2:611–4. DOI: 10.1016/j.jaccas.2020.01.007. PMID: 32432226. PMCID: PMC7236804.
9. Tanabe Y, Itoh E, Suzuki K, Ito M, Hosaka Y, Nakagawa I, et al. 2002; Limited role of coronary angioplasty and stenting in coronary spastic angina with organic stenosis. J Am Coll Cardiol. 39:1120–6. DOI: 10.1016/S0735-1097(02)01746-1. PMID: 11923034.
10. Copeland H, Hayanga JW, Neyrinck A, MacDonald P, Dellgren G, Bertolotti A, et al. 2020; Donor heart and lung procurement: a consensus statement. J Heart Lung Transplant. 39:501–17. DOI: 10.1016/j.healun.2020.03.020. PMID: 32503726.
Fig. 1
Electrocardiogram (ECG) during hospitalization. (A) Normal ECG after cardiac transplantation. (B) ECG after the first cardiac arrest, indicating inferior ST-segment elevation myocardial infarction (STEMI). (C) ECG after stent implantation to the right coronary artery with a stabilized ST segment. (D) ECG after the second cardiac arrest, indicating inferior STEMI.
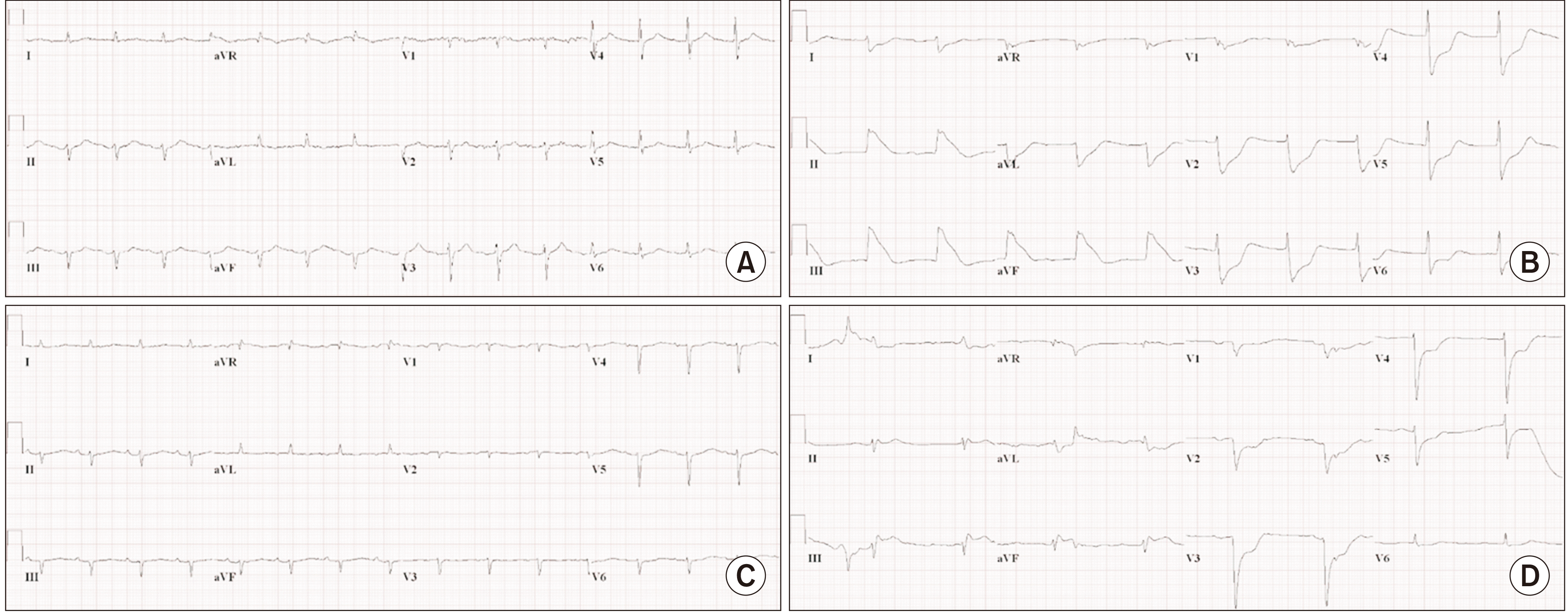
Fig. 2
Coronary angiography (CAG) and intravascular ultrasound after cardiac arrest. (A) CAG of the right coronary artery (RCA) before stent implantation revealed multiple vasospasm. (B) Intravascular ultrasound of the proximal RCA found a large plaque burden (up to 75.5%). (C) A drug-eluting stent was implanted into the proximal RCA. (D) CAG of the RCA after the second cardiac arrest revealed a severe diffuse coronary spasm, except in the area of the stent-implanted proximal lesion.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download