Abstract
Background
The number of end-stage renal disease (ESRD) patients is increasing in Bangladesh. Currently, living kidney donation is the only viable option for transplantation in Bangladesh, and it is further restricted by ABO compatibility issues. We have performed ABO-incompatible kidney transplantations (ABOi KTs) in Bangladesh since 2018. This study examines our experiences with seven cases of ABOi KT.
Methods
The desensitization protocol included low-dose rituximab (100 mg/body) followed by plasma exchange (PEX), which was followed by a 5-g dose of intravenous immunoglobulin. Immunosuppression was undertaken using tacrolimus (0.1 mg/kg/day), mycophenolate mofetil (1,500 mg/day), and prednisolone (0.5 mg/kg/day). All patients received basiliximab for induction therapy.
Results
The median baseline anti-ABO antibody titer was 164 (range, 132–1128). Transplantation was performed at a titer of ≤18. Our patients attended three to five PEX sessions before transplantation. Graft survival was 100% in the seven cases over a mean period of 22 months. The mean creatinine level was 204.6±47.4 µmol/L. Two patients were suspected of having developed acute rejection and received intravenous methylprednisolone, resulting in improved kidney function. One patient required posttransplant hemodialysis due to delayed graft function and subsequently improved. Infection was the most common complication experienced by ABOi KT patients. Two patients developed severe cytomegalovirus pneumonia and died with functioning grafts.
The number of end-stage renal disease (ESRD) patients in Bangladesh increases daily. According to hospital statistics, approximately 35,000 to 40,000 patients in Bangladesh reach ESRD each year. Kidney transplantation is the best renal replacement therapy for ESRD patients. In Bangladesh, kidney transplantations have been conducted since 1982. Unfortunately, only 25% of ESRD patients in Bangladesh can access renal replacement therapy, and, among them, only 2% undergo renal transplantation [1]. A scarcity of kidney donors has worsened this problem. Deceased donor transplantation is not yet performed and live donation is restricted to patients' relatives only. Although ABO-incompatible kidney transplantations (ABOi KTs) are increasingly practiced worldwide to expand the kidney donor pool, reports from developing countries are limited. The greater expense and high risk of acute rejection and life-threatening infection posttransplant are major barriers for ABOi KT in resource-poor countries [2]. Ours is the only center in Bangladesh to have performed ABOi KTs since 2018. In this study, we report our experiences with ABOi KT procedures.
This study was approved by the Institutional Review Board of Kidney Foundation Hospital and Research Institute (IRB No. KFHRI/ECC-001/2022). Informed consent was taken from patients.
This is a retrospective analysis of ABOi KTs performed at the Kidney Foundation Hospital and Research Institute, Dhaka, Bangladesh between June 2018 and September 2019. A total of seven ABOi KTs were performed.
Before transplantation, all donors and recipients underwent a complete blood count; a C-reactive protein test; blood grouping and human leukocyte antigen (HLA) cross-matching; HLA tissue typing; renal, liver, and thyroid function tests; a chest X-ray; an electrocardiogram; an echocardiogram; viral screening (hepatitis B surface antigen, anti-hepatitis C virus, human immunodeficiency virus, QuantiFERON-cytomegalovirus [CMV]); a sexually transmitted infection laboratory test; a urine microscopic examination; and a urine culture. Endoscopy of the upper gastrointestinal tract and color Doppler ultrasonography of the pelvic vessels were also performed on all recipients. In donors, a computed tomographic renal angiogram and diethylenetriamine pentaacetate renogram were performed to evaluate split renal function and renal vasculature.
Immunomodulation: Rituximab (anti-CD20) at a low dose (100 mg/dose) was administered 2 weeks pretransplant. Patients were premedicated with hydrocortisone and pheniramine. Rituximab was diluted with 100 mL of 0.9% normal saline and administered over 4 hours. Intravenous immunoglobulin (IVIG) was administered at a low dose (5 g/dose) after each plasma exchange (PEX) session. A total dose of 15 to 25 g was administered.
Antibody depletion: single-filter PEX was conducted to achieve isoagglutinin (IgG) removal and was begun 1 week pretransplant. We used a COM.TEC machine (Fresenius Kabi, Bad Homburg vor der Höhe, Germany) for PEX. The number of PEX sessions was determined by measuring the serial antibody titer. The volume of plasma exchanged was 30 mL/kg, and the replacement fluid was fresh frozen plasma (FFP) from the donors’ blood groups. PEX was conducted on alternate days until the target IgG titer was achieved. In our ABOi cases, the highest baseline titer was 1:128, and the titer needed to conduct transplantation was ≤1:8. No patient received PEX posttransplant.
Induction: basiliximab (20 mg on day 0 and posttransplant day 4) was given to all patients. Intravenous methylprednisolone was not administered to any patients for induction. Immunosuppression: immunosuppression was conducted using tacrolimus (0.1 mg/kg/day), mycophenolate mofetil (1,500 mg/day), and prednisolone (20 mg/day), which were administered 2 weeks prior to transplantation. The desensitization protocol is shown in Fig. 1.
The anti-ABO IgG titer was measured through a conventional tube test (indirect antiglobulin test). The IgG titer was checked on alternating days pretransplantation and on the day of transplantation. The posttransplantation IgG titer was monitored three times over the course of the first posttransplant week, two times during the following week, and then whenever renal function worsened. HLA crossmatching was performed using the complement-dependent cytotoxicity test.
Triple immunosuppression was continued posttransplantation. The target tacrolimus trough was 8 to 12 ng/mL for the first 3 months, 6 to 8 ng/mL for the next 3 months, and 4 to 6 ng/mL after 6 months. Mycophenolate mofetil was administered at a dose of 1,500 mg/day. Posttransplantation prednisolone was administered at a dose of 0.5 mg/kg/day, then reduced to 20 mg after 1 month, after which the dose was gradually decreased to a maintenance dose of 5 mg/day.
Posttransplantation, all patients were given sulfamethoxazole (400 mg) and trimethoprim (80 mg) once daily for 6 months for prophylaxis against Pneumocystis jirovecii pneumonia. CMV prophylaxis (valganciclovir according to estimated glomerular filtration rate) was administered for 3 months in situations in which either the donor or recipient was QuantiFERON-CMV reactive. A QuantiFERON-CMV enzyme-linked immunosorbent assay was used to detect interferon gamma. Routine anti-tuberculosis or antifungal prophylaxis medications were not given. No biopsy protocol was included; biopsy was performed only when indicated clinically. CD19 or CD20 cell counts were not monitored.
A total of seven ABOi KT procedures were performed. Six of the recipients were men (86%) and one was a woman (14%). The mean age of the recipients was 27 years (range, 22–36 years). One donor was a man (14%) and six were women (86%). The mean age of the donors was 49 years (range, 39–61 years). Six out of the seven donations were from a mother to her child, and one was from a sibling (brother). Transplantation was performed at a titer of ≤1:8. The ABOi KT was performed with an A-positive donor to an O-positive recipient in three cases, a B-positive donor to an O-positive recipient in two cases, an A-positive donor to a B-positive recipient in one case, and an AB-positive donor to a B-positive recipient in one case. The basic demographics of donors and recipients are shown in Table 1.
The median baseline anti-ABO antibody titer was 1:64 (range, 1:32–1:128). Graft survival across all seven cases was 100% over a mean follow-up period of 22 months. The longest follow-up period was 76 months. The mean creatinine level was 204.6±47.4 µmol/L (Table 2). After transplantation, the average anti-ABO antibody titer was 1:4 (range, 1:2–1:8).
During the desensitization period, patient 1 developed a chest infection that was treated empirically with intravenous antibiotics. No organism was isolated. Transplantation was performed with antibiotic coverage. Patient 7 developed ST-elevation myocardial infarction during the third PEX session, which was managed conservatively with dual antiplatelets and low-molecular-weight heparin. Over the next 3 weeks, no further PEX was performed and the anti-ABO IgG titer remained at 1:8. Transplantation proceeded, and the postoperative period was uneventful.
During the immediate postoperative period, two patients developed accelerated hypertension that was managed with intravenous antihypertensives (labetalol, glyceryl trinitrate), and one patient developed subacute intestinal obstruction, which resolved with conservative management.
Two patients experienced rising serum creatinine posttransplant. Acute rejection was suspected, and it was treated with intravenous methylprednisolone, which resulted in partial improvement in kidney function. Two patients developed gastrointestinal disorders during the postoperative period, which were managed conservatively. Patient 2 developed acute psychosis 3 weeks posttransplant, which was managed with antipsychotic medications. Patient 5 required hemodialysis posttransplant due to delayed graft function, which was believed to be caused by acute tubular injury due to intraoperative bleeding and hypotension. After multiple hemodialysis sessions, the graft function recovered.
Infection was the most common complication among the ABOi KT patients in this study. Two patients (patient 3 and patient 6) developed severe pneumonia (suspected CMV pneumonia) and died with a functioning graft. Both patient 3 and patient 3’s donor were QuantiFERON-CMV-positive, and the patient was treated with prophylactic valganciclovir. Beginning on postoperative day 17, the patient received a 500-mg injection of methylprednisolone for 3 consecutive days due to suspected acute rejection. Three months later, the patient developed left upper lobe pneumonia. Since the patient had stopped taking valganciclovir, CMV pneumonia was suspected. Due to financial constraints, the cause of pneumonia could not be evaluated. He was treated with oral valganciclovir and empirical antibiotics. The patient gradually improved and was discharged. Two months later, the patient was again admitted with bilateral pneumonia with a serum creatinine level of 275 µmol/L. The patient had not been taking oral valganciclovir at home. He began treatment with intravenous ganciclovir and was moved to the intensive care unit. Unfortunately, the patient died several days later.
Both patient 6 and patient 6’s donor were QuantiFERON-CMV reactive, and the patient began to receive treatment with prophylactic valganciclovir. The patient did not receive any anti-rejection therapy. Six weeks later, he was admitted to the hospital after 1 week of respiratory distress, cough, and fever. A chest X-ray revealed several bilateral patchy consolidations. The patient had been taking oral valganciclovir irregularly. CMV pneumonia was suspected. However, the patient developed respiratory failure and was referred to the intensive care unit of another hospital. Unfortunately, he died within 48 hours. The complications experienced by patients are shown in Table 3.
ABOi KT has substantially expanded the living kidney donor pool worldwide. In Bangladesh, the number of ESRD patients is increasing exponentially; however, the number of kidney transplantations performed each year is not increasing, and the waiting list of transplant candidates is growing. There are several causes for this. For example, there is a shortage of suitable living related donors, the deceased donor transplant program has not yet begun, and unrelated live donation and kidney swaps are not allowed by law. In this context, ABOi KT poses a viable alternative to increase the number of transplantations.
Rituximab is an integral part of desensitization during ABOi KT. Most centers use rituximab at a dose of 375 mg/m2 of body surface area. However, Ray et al. [3] and Shirakawa et al. [4] used a low-dose of rituximab at 200 mg/dose and reported excellent graft and patient survival. Fuchinoue et al. [5] administered rituximab at different doses (100 mg, 200 mg, and 500 mg) to ABOi KT recipients, and at 1-year posttransplantation, the graft survival rate was 100% for all three groups. Habicht et al. [6] reported an increased incidence of infectious complications with the standard dose of rituximab (375 mg/m2). Lee et al. [7] demonstrated that fewer infectious complications occurred with a low dose of rituximab (200 mg) than with the standard dose, and the graft outcomes were similar. At our center, seven ABOi KTs were performed using a low dose of rituximab (100 mg). We did not experience any hyperacute rejections or graft loss over the course of the longest follow-up period of 76 months. However, serum creatinine levels in all our patients were higher than normal.
As a replacement fluid during PEX, we used FFP, which is a cost-effective alternative to albumin and is, therefore, preferred. In addition, the risk of bleeding is lower with FFP. We did not experience any bleeding complications during PEX therapy in our patients. However, the use of FFP can cause anaphylaxis, hypotension, citrate-associated paraesthesia, urticaria, and blood-borne infections [8]. Our patients did not experience any of the above problems. Notably, patient 7 developed myocardial infarction after PEX, which is a rarely reported but life-threatening complication of PEX [9]. The cause of the myocardial infarction could not be established due to financial constraints. However, given the age of the patient, coronary atherosclerosis was unlikely. The suspected causes of the acute myocardial infarction were a coronary artery spasm or a coronary artery embolism. Other causes of acute myocardial infarction during PEX, such as hypotension or arrhythmia, were not observed in our patient. In our patients, we administered 5 g of IVIG after each PEX session, which is less than the protocols at most other centers and is a more cost-effective regimen. Ray et al. [3] also administered 5 g of IVIG after each PEX session.
Death due to infection with a functioning graft is common in developing countries [10]. In Bangladesh, posttransplant infection is more common [11]. CMV pneumonia is a common cause of mortality in renal transplant recipients, with a reported fatality rate of 65% to 90% [12]. Two of our ABOi KT patients passed away due to suspected CMV pneumonia. Both of the patients and donors were QuantiFERON-CMV-positive. One patient had stopped taking prophylactic valganciclovir, while the other patient was taking valganciclovir irregularly and presented late in the hospital with respiratory failure. Although CMV is found all across the world, it is more prevalent in developing countries [13]. A study on allogeneic stem cell transplant patients at a single center in Pakistan demonstrated that the incidence of CMV infection was 18.2%, and the incidence of CMV disease was 3.9% with 100% mortality [14]. Another study from India reported a high incidence of CMV disease (19.3%) [15].
Recurrent episodes of infectious complications during the pre- and posttransplant period were the major hurdles we faced when performing ABOi KT in our country. Other studies also reported that the overall infectious disease burden is higher in low middle-income countries than in high-income countries [15]. The suspected causes behind this include high population density, poverty, malnutrition, and poor sanitation and housing facilities. In developing countries such as Bangladesh, patients receiving kidney transplantation often come from poor socioeconomic backgrounds and do not have healthcare coverage. Frequent hospitalizations due to posttransplant infections can result in a loss of employment and further loss of income, accompanied by additional out-of-pocket expenses for treatment. This is one of the major causes of death and graft loss in these patients [15]. Very close monitoring of patients; instructions to avoid crowds; improved nutrition, housing, and sanitary conditions; and early notification and prompt treatment of even mild infections may be potential solutions to this issue. Above all, strengthening the economic status of patients along with government subsidies and financial assistance from non-government organizations are needed to improve overall outcomes.
Although none of our patients experienced hyperacute rejections, episodes of suspected acute and chronic rejection were observed. Acute and chronic rejection in our patients were clinically suspected. Unfortunately, we managed to conduct a renal biopsy in one patient only, and the other patients were either not fit to undergo a biopsy or did not agree to receive one. Treatment for those rejection episodes was limited by the presence of simultaneous major infections.
All our patients received living related kidney transplantation. Although none of our patients experienced graft loss, the serum creatinine levels in all of our patients were quite high. There could be several possible reasons behind this. First, to avoid infection, we used a low-dose immunosuppression protocol. The dose of rituximab (100 mg/day) we used was lower than that used at most international centers [3,6], and induction therapy included only basiliximab, whereas most other centers have used methylprednisolone, anti-thymocyte globulin, or both in addition to basiliximab [2]. No facilities in Bangladesh perform donor-specific antibody or panel reactive antibody tests. Therefore, the immunosuppression protocol was generalized rather than individualized. Second, all our patients (except for patients 6 and 7) experienced postoperative complications (Table 3) that might have influenced the outcomes related to the transplanted kidney. Third, patients typically exhaust all of their resources in order to undergo a transplantation procedure. Hence, after transplantation, the patients in this study could barely afford basic medications and were sometimes unable to pay for additional medications, tests, or hospitalizations. As a result, not every episode of acute rejection could be properly evaluated, and management was often limited to affordable medications only.
In our experience, the high cost of ABOi KT is another major barrier to performing the procedure. Our center is a private non-profit organization. The cost of ABO-compatible KT at our center is around $3,000, and it is approximately $1,900 at public hospitals and $9,900 at private hospitals. Ours is the only center in Bangladesh that performs ABOi KT, and the cost of ABOi KT is $9,000, even with our cost-effective preconditioning protocol. For a large number of ESRD patients in Bangladesh, it is often not possible to spend an extra $6,000 for the procedure and, therefore, cannot undergo ABOi KT. Although sometimes part of this cost is funded by a hospital trust, financial support from the government could substantially mitigate this barrier.
The introduction of ABOi KTs in Bangladesh is a major step forward towards expanding the breadth of Bangladesh’s transplantation facilities. This gives hope to a large number of ESRD patients who do not have ABO-compatible donors. However, the risks of rejection and infection posttransplant are major concerns that require special attention. Extensive counselling and discussions with patients regarding pre- and posttransplantation expenses and ways to prevent complications might be helpful. In addition, strengthening the economic status of patients along with government subsidies and financial assistance from non-government organizations are needed to make ABOi KT more affordable and increase the likelihood of success.
ACKNOWLEDGMENTS
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Funding/Support
This study was supported by research grant from the Kidney Foundation Hospital and Research Institute, Dhaka, Bangladesh (grant No. KFHRI 000-00-078).
Author Contributions
Conceptualization: all authors. Data curation: NASB, TSK. Formal analysis: all authors. Funding acquisition: HUR. Methodology: all authors. Project administration: NASB, HUR. Visualization: NASB, HUR. Writing–original draft: NASB. Writing–review & editing: all authors.
REFERENCES
1. Rashid HU, Alam MR, Khanam A, Rahman MM, Ahmed S, Mostafi M, et al. Moura-Neto JA, Divino-Filho JC, Ronco C, editors. 2021. Nephrology in Bangladesh. Nephrology Worldwide. Springer;Cham: p. 221–38. DOI: 10.1007/978-3-030-56890-0_18.
2. Nepali R, Shah DS. 2019; Experience of starting ABO incompatible renal transplant in Nepal. Transpl Rep. 4:100026. DOI: 10.1016/j.tpr.2019.100026.
3. Ray DS, Thukral S. 2016; Outcome of ABO-incompatible living donor renal transplantations: a single-center experience from eastern India. Transplant Proc. 48:2622–8. DOI: 10.1016/j.transproceed.2016.06.048. PMID: 27788792.
4. Shirakawa H, Ishida H, Shimizu T, Omoto K, Iida S, Toki D, et al. 2011; The low dose of rituximab in ABO-incompatible kidney transplantation without a splenectomy: a single-center experience. Clin Transplant. 25:878–84. DOI: 10.1111/j.1399-0012.2010.01384.x. PMID: 21175849.
5. Fuchinoue S, Ishii Y, Sawada T, Murakami T, Iwadoh K, Sannomiya A, et al. 2011; The 5-year outcome of ABO-incompatible kidney transplantation with rituximab induction. Transplantation. 91:853–7. DOI: 10.1097/TP.0b013e31820f08e8. PMID: 21297552.
6. Habicht A, Bröker V, Blume C, Lorenzen J, Schiffer M, Richter N, et al. 2011; Increase of infectious complications in ABO-incompatible kidney transplant recipients: a single centre experience. Nephrol Dial Transplant. 26:4124–31. DOI: 10.1093/ndt/gfr215. PMID: 21622990.
7. Lee J, Lee J, Kim S, Ju M, Joo D, Kim M, et al. 2015; Infectious complications in ABO incompatible kidney transplantation recipient according to the rituximab dose [abstract]. Am J Transplant. 15(suppl 3):C88.
8. McLeod BC. 2006; Therapeutic apheresis: use of human serum albumin, fresh frozen plasma and cryosupernatant plasma in therapeutic plasma exchange. Best Pract Res Clin Haematol. 19:157–67. DOI: 10.1016/j.beha.2005.01.004. PMID: 16377548.
9. Ziselman EM, Bongiovanni MB, Wurzel HA. 1984; The complications of therapeutic plasma exchange. Vox Sang. 46:270–6. DOI: 10.1111/j.1423-0410.1984.tb00086.x. PMID: 6730424.
10. Yu MY, Kim YC, Lee JP, Lee H, Kim YS. 2018; Death with graft function after kidney transplantation: a single-center experience. Clin Exp Nephrol. 22:710–8. DOI: 10.1007/s10157-017-1503-9. PMID: 29159528. PMCID: PMC5956041.
11. Mitra P, Hossain MG, Hossan ME, Khoda MM, Rahim MA, Hossain MJ, et al. 2018; A decade of live related donor kidney transplant: experience in a tertiary care hospital of Bangladesh. BIRDEM Med J. 8:198–202. DOI: 10.3329/birdem.v8i3.38121.
12. Dong B, Wang Y, Wang G, Wang W, Zhou H, Fu Y. 2014; A retrospective study of cytomegalovirus pneumonia in renal transplant patients. Exp Ther Med. 7:1111–5. DOI: 10.3892/etm.2014.1577. PMID: 24940395. PMCID: PMC3991547.
13. Chakravarti A, Kashyap B, Matlani M. 2009; Cytomegalovirus infection: an Indian perspective. Indian J Med Microbiol. 27:3–11. DOI: 10.1016/S0255-0857(21)01744-8. PMID: 19172051.
14. Ullah K, Raza S, Ahmed P, Chaudhry QU, Satti TM, Ahmed S, et al. 2008; Post-transplant infections: single center experience from the developing world. Int J Infect Dis. 12:203–14. DOI: 10.1016/j.ijid.2007.06.012. PMID: 17920999.
15. Gopalakrishnan V, Agarwal SK, Aggarwal S, Mahajan S, Bhowmik D, Bagchi S. 2019; Infection is the chief cause of mortality and non-death censored graft loss in the first year after renal transplantation in a resource limited population: a single centre study. Nephrology (Carlton). 24:456–63. DOI: 10.1111/nep.13401. PMID: 29761588.
Fig. 1
Desensitization protocol. TAC, tacrolimus; MMF, mycophenolate mofetil; PEX, plasma exchange; IVIG, intravenous immunoglobulin; TX, transplant.
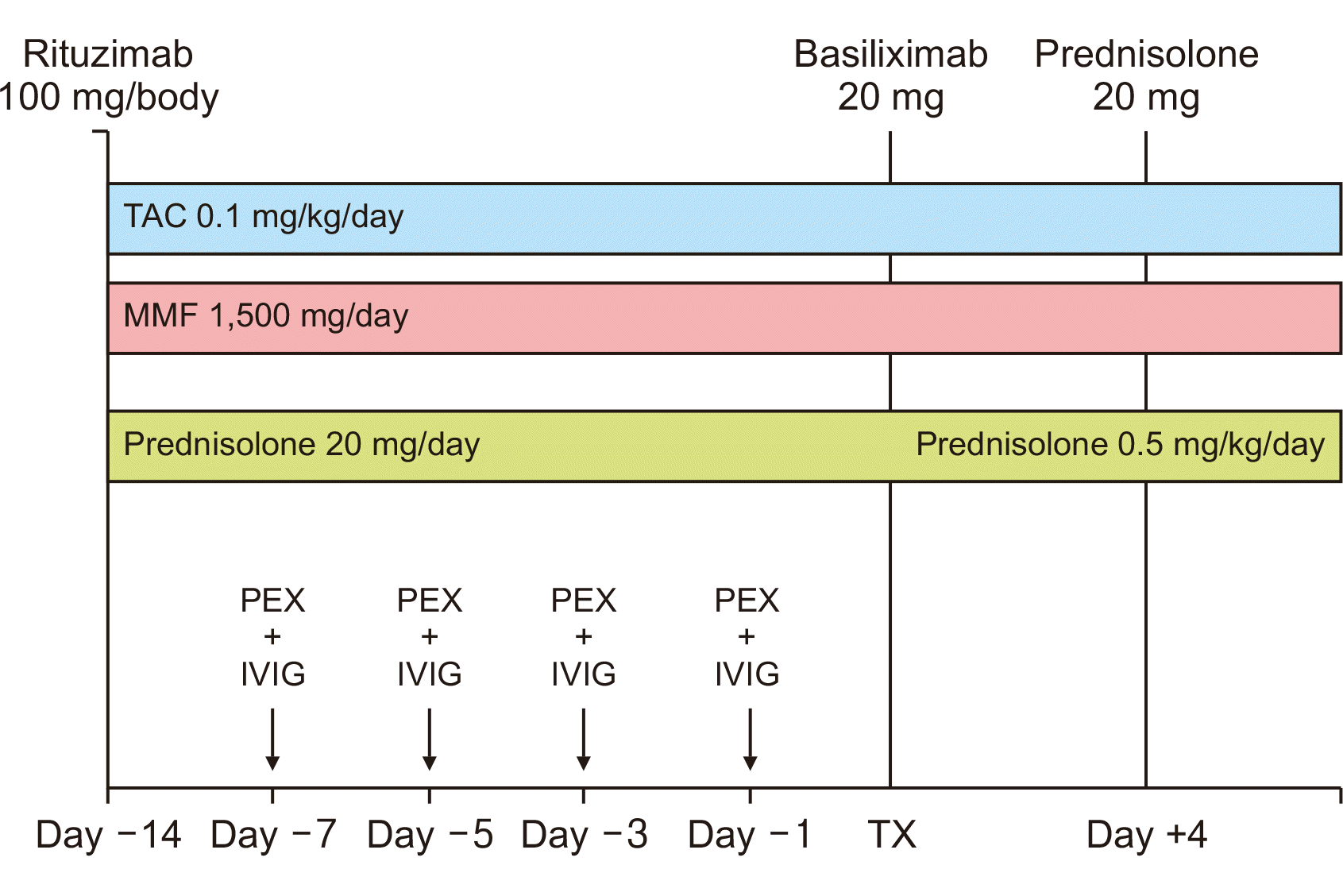
Table 1
Basic demographics of donors and recipients
| Patient number | Age | Sex | Blood type | Donor relation | HLAa) mismatch | Native renal disease | Donor DTPA GFR (mL/min) | Duration of HD before transplant (mo) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||
| Donor | Recipient | Donor | Recipient | Donor | Recipient | ||||||||
| 1 | 52 | 23 | F | M | A | O | Mother | 2/4 | CGN | 77 | 3 | ||
| 2 | 50 | 36 | F | M | A | O | Mother | 2/4 | CGN | 95 | 11 | ||
| 3 | 54 | 30 | F | M | A | O | Mother | 2/4 | CGN | 82 | 7 | ||
| 4 | 52 | 24 | F | F | B | O | Mother | 2/4 | IgA | 77 | 5 | ||
| 5 | 39 | 22 | F | M | B | O | Mother | 2/4 | CGN | 83 | 3 | ||
| 6 | 61 | 26 | F | M | AB | B | Mother | 1/4 | CGN | 79 | 48 | ||
| 7 | 36 | 28 | M | M | A | B | Brother | 0/4 | FSGS | 118 | 5 | ||
Table 2
Desensitization protocol and outcomes
Table 3
Complications of the recipients




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download