Abstract
Background
This is the first report on three-dimensional (3D) laparoscopic donor nephrectomy performed in the Central Asian region and Commonwealth of Independent States countries. This study presents the results of our initial experiences of 3D hand-assisted laparoscopic donor nephrectomy (3D-HALDN) in comparison with the outcomes of two-dimensional hand-assisted laparoscopic donor nephrectomy (2D-HALDN) at a single center.
Methods
From 2015 to 2019, 19 3D-HALDN and 19 2D-HALDN procedures were performed at the same center by two surgeons. All 38 procedures used identical techniques. Between-group differences were considered statistically significant at P<0.05.
Results
The baseline characteristics in both groups were statistically comparable (P>0.05). All donors underwent left nephrectomy. Donors who underwent 3D-HALDN had better outcomes than those who underwent 2D-HALDN, as shown by a shorter warm ischemic time (P<0.05), a shorter operative time (P<0.05), and less blood loss (P<0.05). There were no conversions or major complications (according to the Clavien-Dindo classification) in either group. The average drainage duration and postoperative hospitalization were significantly shorter in the 3D-HALDN group (P<0.05). The between-group differences in the mean postoperative creatinine level and glomerular filtration rate were not significant.
Conclusions
The 3D-HALDN approach is more beneficial than traditional 2D-HALDN by providing a shorter warm ischemic time, less blood loss, and shorter durations of drainage and postoperative hospitalization. Postoperative complications and the functional condition of the kidney in donors in the early and late postoperative periods did not depend on the type of laparoscopic donor nephrectomy.
After the introduction of laparoscopic donor nephrectomy into clinical practice by Ratner and co-authors in 1995, it gained popularity and over time has become one of the main surgical procedures performed in living kidney donors [1,2]. In 1998, the first report of laparoscopic nephrectomy with manual assistance was published, with encouraging results [3]. Many recent studies have shown that laparoscopic nephrectomy has clinical, emotional, psychological, and economic advantages compared to open surgery [4,5].
All types of minimally invasive nephrectomy in living donors have undeniable advantages over the open technique, but they have not shown significant differences in comparison with each other [6]. In the last few years, data have been published on the usage of three-dimensional (3D) imaging for living donor nephrectomy [7-10]. The purpose of this report was to present the results of the initial experiences of 3D hand-assisted laparoscopic donor nephrectomy (3D-HALDN) at a single transplant center and compare them with traditional two-dimensional hand-assisted laparoscopic donor nephrectomy (2D-HALDN) with manual assistance. This is the first report on a series of 3D-HALDN procedures performed in the Central Asian region and Commonwealth of Independent States countries.
Our study was performed using clinical data with the approval of the Institutional Review Board of West Kazakhstan Medical University (IRB No. 44A of August 2, 2021). All patients were informed, consents were taken, there were no refusals.
From February 2017 to July 2019, two surgeons performed 3D-HALDN in 19 donors. The medical records of these patients were analyzed according to the following data: (1) age and sex, (2) relationship to the recipient, (3) body mass index (BMI), (4) nephrectomy side, (5) operative time (the duration from the beginning of the first incision to the last suture of the wound), (6) warm ischemic time (the duration from the clamping of the renal artery to the transfer of the organ to the back-table), (7) preoperative serum creatinine and estimated glomerular filtration rate (GFR) by modification of diet in renal disease (MDRD), (8) postoperative serum creatinine and estimated GFR by MDRD on days 1, 6, and 30 after HALDN, and (9) duration of drainage and hospitalization stay after surgery.
The Clavien-Dindo classification system was used to assess postoperative complications in donors. To conduct a comparative study with a second group of patients, 19 left-sided 2D-HALDN cases performed in the same center by the same two surgeons were retrospectively analyzed. All potential donors underwent examinations before transplantation according to the approved local clinical protocols. These examinations included anamnesis collection, a physical examination, determination of blood tests, evaluation of GFR, intravenous angiography and urography with 3D spiral computed tomography.
The surgical technique of HALDN was identical in all 38 patients. In both groups, the Thunderbeat device (Olympus Europa, Hamburg, Germany) was used for dissection. After general anesthesia, the donors were placed on the operating table in a lateral position. An upper-median laparotomy with a length of 7 cm was performed with the installation of an assistant hand-port for the surgeon’s left hand. Then, two additional ports were inserted into the abdomen for the camera and the manipulation instruments (Fig. 1). Next, the kidney was mobilized according to the standard procedure we have described earlier [10]. After clipping and cutting the ureter and renal vessels, the kidney graft was extracted through an open gel-port. If necessary, a drain was left through the wound of the working trocar.
To compare differences in the median values of variables between the two groups, the Student t-test and the Mann-Whitney U-test were used. Differences between the two groups were considered significant at P<0.05.
From February 2017 to July 2019, two surgeons at one center performed 3D-HALDN in 19 donors, and 2D-HALDN in 19 donors between 2015 and 2019. All donors in both groups were relatives of recipients, and all of them underwent left nephrectomy. Of the 19 patients who underwent 3D-HALDN, 12 (63%) were men, seven (37%) were women, and the average age was 41±10.9 years. In 3D-HALDN group, four patients (21%) had two renal arteries. Table 1 shows a comparison of the preoperative demographic data of donors before 2D- and 3D-HALDN. The baseline characteristics of the donors in both groups were statistically comparable (P>0.05). According to the World Health Organization, an adult BMI of 30 kg/m2 or more is considered to indicate obesity. Thus, our donors were divided according to whether their BMI was <30 or ≥30 kg/m2 to facilitate a reliable comparison of statistical data.
The intraoperative data of donors in both groups are shown in Table 2. Compared to the donors who underwent 2D-HALDN, those who underwent 3D-HALDN had a shorter average warm ischemic time (116±11.2 vs. 126±13.1 seconds, P=0.02), a shorter operative time (182.4±37.0 vs. 210.5±46.8 minutes, P<0.05), and a lower volume of blood loss (42±34.4 vs. 71±34.6 mL, P=0.01). There were no conversions in either group.
In both groups, there were no major postoperative complications according to the Clavien-Dindo classification (P>0.05). Grade I complications occurred in the form of prolonged lymphorrhea through drainage (3D-HALDN, one case; 2D-HALDN, one case) and postoperative ileus (2D-HALDN, two cases). These complications did not require active intervention. The average duration of drainage and the postoperative hospital stay were significantly shorter in the 3D-HALDN group than in the 2D-HALDN group (3.0±0.6 vs. 3.6±0.6 days, and 6.0±1.0 vs. 7.0±1.2 days, respectively; P<0.05). The mean creatinine level and GFR on days 1, 6, and 30 did not show statistically significant differences between the two groups of donors (Table 3).
Laparoscopic donor nephrectomy has undeniable advantages over traditional open surgery and has become the gold standard at many transplant centers [6,11-13]. The transition to 3D laparoscopic donor nephrectomy was made due to the previous introduction of 3D imaging in clinical surgery [13,14]. The organ transplantation program at our center started in 2014. HALDN operations have been performed since 2015, and since 2017, laparoscopy with 3D visualization has been used for this procedure. All 3D-HALDN cases included in this study were performed by two surgeons, and for an adequate comparison, the data of 19 donors with 2D-HALDN performed by the same surgeons were retrospectively analyzed. Both surgeons had experience performing traditional 2D laparoscopic surgery (including nephrectomy) and open donor nephrectomy before switching to 3D-HALDN. This approach is somewhat consistent with the data of Schoenthaler et al. [15], who found no differences in experienced surgeons’ techniques of performing 2D and 3D laparoscopy on phantoms.
Over the past few years, comparative studies have been published showing various advantages of 3D laparoscopic operations over 2D operations, both for educational purposes and in practical surgery [16-19]. Some researchers have pointed out the obvious superiority of 3D laparoscopy over 2D laparoscopy for complex surgery (e.g., oncological, urological, and bariatric surgery) [19-21]. However, some randomized studies revealed no differences in the performance of cholecystectomy, hernioplasty, or appendectomy between these two laparoscopic methods [22,23]. We consider laparoscopic donor nephrectomy to be one of the most complex operations; moreover, in this procedure, healthy donors with altruistic motivations are subjected to surgical interventions and risks without any expected therapeutic effects. Other recent studies have shown the advantages of robotic laparoscopic donor nephrectomy over traditional 2D laparoscopic donor nephrectomy [24,25]. At the same time, Mulder et al. [8], in a series of 40 operations, showed clinical and economic benefits of using 3D laparoscopic donor nephrectomy before robotic nephrectomy. Achit et al. [26] revealed the cost-effectiveness of hand-assisted laparoscopic nephrectomy in comparison with open, standard laparoscopic and robotic nephrectomy. Therefore, we consider it acceptable to use 3D visualization for nephrectomy in living donors.
According to the 2018 European Association for Endoscopic Surgery consensus, 3D laparoscopy reduces the time of surgery, but identifying its benefits in relation to complications would require standardization and methodological uniformity in further clinical trials [27]. In our study, the demographic and clinical baseline characteristics of donors before surgery in both groups were comparable (P>0.05). Both HALDN approaches were performed by two surgeons in the same operating room, using identical surgical equipment and instruments for tissue dissection and vascular and ureter ligation, with the exception of the video camera used for visualization. Our analysis of parameters including the duration of the operation, intraoperative blood loss, and the duration of drainage and postoperative hospital stay showed 3D-HALDN to be advantageous over 2D-HALDN (P<0.05). When comparing complications and conversions between the two groups, no statistically significant difference was found (P>0.05). In one patient in the 3D-HALDN group, prolonged lymphorrhea through drainage was observed for up to 5 days. In the 2D-HALDN group, lymphorrhea by drainage occurred in one case and non-prolonged ileus was noted in two cases. These complications did not require any active intervention.
In the available literature, we found only one study that compared 3D and 2D laparoscopic donor nephrectomy [28]. Therefore, we believe that the number of such articles is not enough to show statistically meaningful differences. Our findings are similar to the data of that study, in that the duration of surgery, the warm ischemic time, and postoperative hospital stay were significantly better in the 3D-HALDN group. Similarly, there were no statistically significant differences in donors’ postoperative complications, creatinine levels, or GFR.
A possible explanation for the significant difference in the operative time may be differences in the required technical skills. Nevertheless, the outcomes in the postoperative period were statistically similar, and the duration of the operation did not affect postoperative complications. In our study, we included the drainage duration for comparison, and we also showed more favorable results in the 3D-HALDN group. We believe that the drainage duration in our donors directly affected the duration of postoperative stay, with relatively similar rates of complications, creatinine levels, and GFR. On average, less intraoperative blood loss was observed in the 3D-HALDN group. It seems that better visualization with depth perception using 3D stereoscopy enables a more thorough hemostatic and lymphostatic technique during surgery. Nevertheless, in the early and late postoperative periods, the function of the single kidney in all donors, regardless of the HALDN method (3D or 2D), remained equally satisfactory.
The principal limitation of this study is the small number of patients for comparison, although we do not think that the small number of cases itself poses a substantial problem. Nonetheless, an analysis of a large population of randomized cases would be necessary to establish the reliability of the benefits of 3D-HALDN.
The results of this study showed that 3D-HALDN had advantages over traditional 2D-HALDN in terms of shortening the warm ischemic time, reducing the volume of blood loss, and shortening the duration of drainage and the postoperative hospitalization stay. However, postoperative complications and the functional status—in terms of the creatinine level and GFR—of donor kidneys in the early and late postoperative periods did not depend on the type of laparoscopic nephrectomy performed.
ACKNOWLEDGMENTS
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: BZ, YS. Formal analysis: NM, DY, SS. Writing–original draft: MR, AY. Writing–review & editing: NM.
Additional Contributions
The authors are grateful to Dr. Sedat Yıldırım, MD, Professor of Surgery, Başkent University School of Medicine for editorial assistance and final review of the manuscript.
REFERENCES
1. Ratner LE, Ciseck LJ, Moore RG, Cigarroa FG, Kaufman HS, Kavoussi LR. 1995; Laparoscopic live donor nephrectomy. Transplantation. 60:1047–9. DOI: 10.1097/00007890-200107270-00039. PMID: 32721258.
2. Branco AW, Kondo W, Branco Filho AJ, George MA, Rangel M, Stunitz LC. 2008; A comparison of hand-assisted and pure laparoscopic techniques in live donor nephrectomy. Clinics (Sao Paulo). 63:795–800. DOI: 10.1590/S1807-59322008000600015. PMID: 19061003. PMCID: PMC2664281.
3. Wolf JS Jr, Tchetgen MB, Merion RM. 1998; Hand-assisted laparoscopic live donor nephrectomy. Urology. 52:885–7. DOI: 10.1016/S0090-4295(98)00389-6. PMID: 15206504.
4. Simforoosh N, Soltani MH, Basiri A, Tabibi A, Gooran S, Sharifi SH, et al. 2014; Evolution of laparoscopic live donor nephrectomy: a single-center experience with 1510 cases over 14 years. J Endourol. 28:34–9. DOI: 10.1089/end.2013.0460. PMID: 24074354.
5. Hung CJ, Lin YJ, Chang SS, Chou TC, Lee PC. 2009; Development of laparoscopic donor nephrectomy: a strategy to increase living kidney donation incentive and maintain equivalent donor/recipient outcome. J Formos Med Assoc. 108:135–45. DOI: 10.1016/S0929-6646(09)60044-9. PMID: 19251549.
6. Shockcor NM, Sultan S, Alvarez-Casas J, Brazio PS, Phelan M, LaMattina JC, et al. 2018; Minimally invasive donor nephrectomy: current state of the art. Langenbecks Arch Surg. 403:681–91. DOI: 10.1007/s00423-018-1700-3. PMID: 30132134.
7. Gamé X, Binhazzaa M, Soulié M, Kamar N, Sallusto F. 2017; Three-dimensional laparoscopy for living-donor nephrectomy with vaginal extraction: the first case. Int J Surg Case Rep. 34:87–9. DOI: 10.1016/j.ijscr.2017.03.025. PMID: 28376420. PMCID: PMC5379907.
8. Mulder EE, Janki S, Terkivatan T, Klop KW, IJzermans JN, Tran TC. 2018; 3D Endoscopic donor nephrectomy versus robot-assisted donor nephrectomy: a detailed comparison of 2 prospective cohorts. Transplantation. 102:e295–300. DOI: 10.1097/TP.0000000000002130. PMID: 29461442.
9. Kumar A, Gupta P, Kumar S, Yadav S, Prasanth YM, Tyagi V, et al. 2019; A prospective evaluation of donor and graft outcomes of 3-D laparoscopic donor nephrectomy: a single centre experience. Cent European J Urol. 72:72. DOI: 10.5173/ceju.2019.1850. PMID: 31011446. PMCID: PMC6469007.
10. Rysmakhanov M, Yelemessov A, Mussin N, Sultangereyev Y, Kaliyev A, Tezcaner T, et al. 2020; Pure 3-dimensional laparoscopic living-donor nephrectomy: first case in Kazakhstan. Exp Clin Transplant. 18(Suppl 1):68–9. DOI: 10.6002/ect.TOND-TDTD2019.P12. PMID: 32008499.
11. Carrión DM, Gómez Rivas J, Aguilera Bazán A, Alonso Y Gregorio S, De Castro Guerín C, Álvarez-Maestro M, et al. 2019; Laparoscopic donor nephrectomy versus open donor nephrectomy: outcomes from a single transplant center. Arch Esp Urol. 72:508–14. PMID: 31223128.
12. Vernadakis S, Marinaki S, Darema M, Soukouli I, Michelakis IE, Beletsioti C, et al. 2021; The evolution of living donor nephrectomy program at a hellenic transplant center. Laparoscopic vs. open donor nephrectomy: single-center experience. J Clin Med. 10:1195. DOI: 10.3390/jcm10061195. PMID: 33809339. PMCID: PMC8001196.
13. Sahu D, Mathew MJ, Reddy PK. 2014; 3D laparoscopy - help or hype; initial experience of a tertiary health centre. J Clin Diagn Res. 8:NC01–3. DOI: 10.7860/JCDR/2014/8234.4543. PMID: 25177597. PMCID: PMC4149103.
14. Zdichavsky M, Schmidt A, Luithle T, Manncke S, Fuchs J. 2015; Three-dimensional laparoscopy and thoracoscopy in children and adults: a prospective clinical trial. Minim Invasive Ther Allied Technol. 24:154–60. DOI: 10.3109/13645706.2014.968171. PMID: 25345416.
15. Schoenthaler M, Schnell D, Wilhelm K, Schlager D, Adams F, Hein S, et al. 2016; Stereoscopic (3D) versus monoscopic (2D) laparoscopy: comparative study of performance using advanced HD optical systems in a surgical simulator model. World J Urol. 34:471–7. DOI: 10.1007/s00345-015-1660-y. PMID: 26242728.
16. Guanà R, Ferrero L, Garofalo S, Cerrina A, Cussa D, Arezzo A, et al. 2017; Skills comparison in pediatric residents using a 2-dimensional versus a 3-dimensional high-definition camera in a pediatric laparoscopic simulator. J Surg Educ. 74:644–9. DOI: 10.1016/j.jsurg.2016.12.002. PMID: 28039097.
17. Currò G, La Malfa G, Caizzone A, Rampulla V, Navarra G. 2015; Three-dimensional (3D) versus two-dimensional (2D) laparoscopic bariatric surgery: a single-surgeon prospective randomized comparative study. Obes Surg. 25:2120–4. DOI: 10.1007/s11695-015-1674-y. PMID: 25893652.
18. Shaikh AR, Shaikh AA, Abbasi M. 2021; Short term outcomes of three dimensional versus two-dimensional laparoscopic cholecystectomy. Pak J Med Sci. 37:162–6. DOI: 10.12669/pjms.37.1.3721. PMID: 33437270. PMCID: PMC7794142.
19. Lee K, Youn SI, Won Y, Min SH, Park YS, Ahn SH, et al. 2021; Prospective randomized controlled study for comparison of 2-dimensional versus 3-dimensional laparoscopic distal gastrectomy for gastric adenocarcinoma. Surg Endosc. 35:934–40. DOI: 10.1007/s00464-020-07587-4. PMID: 32356108.
20. Dirie NI, Wang Q, Wang S. 2018; Two-dimensional versus three-dimensional laparoscopic systems in urology: a systematic review and meta-analysis. J Endourol. 32:781–90. DOI: 10.1089/end.2018.0411. PMID: 29969912. PMCID: PMC6156697.
21. Padin EM, Santos RS, Fernández SG, Jimenez AB, Fernández SE, Dacosta EC, et al. 2017; Impact of three-dimensional laparoscopy in a bariatric surgery program: influence in the learning curve. Obes Surg. 27:2552–6. DOI: 10.1007/s11695-017-2687-5. PMID: 28456885.
22. Schwab KE, Curtis NJ, Whyte MB, Smith RV, Rockall TA, Ballard K, et al. 2020; 3D laparoscopy does not reduce operative duration or errors in day-case laparoscopic cholecystectomy: a randomised controlled trial. Surg Endosc. 34:1745–53. DOI: 10.1007/s00464-019-06961-1. PMID: 31312963. PMCID: PMC7093411.
23. Botteri E, Ortenzi M, Alemanno G, Giordano A, Travaglio E, Turolo C, et al. 2021; Laparoscopic appendectomy performed by junior surgeons: impact of 3D visualization on surgical outcome. Randomized multicentre clinical trial. (LAPSUS TRIAL). Surg Endosc. 35:710–7. DOI: 10.1007/s00464-020-07436-4. PMID: 32060747.
24. Serni S, Pecoraro A, Sessa F, Gemma L, Greco I, Barzaghi P, et al. 2021; Robot-assisted laparoscopic living donor nephrectomy: the University of Florence technique. Front Surg. 7:588215. DOI: 10.3389/fsurg.2020.588215. PMID: 33521044. PMCID: PMC7844329.
25. Spaggiari M, Garcia-Roca R, Tulla KA, Okoye OT, Di Bella C, Oberholzer J, et al. 2022; Robotic assisted living donor nephrectomies: a safe alternative to laparoscopic technique for kidney transplant donation. Ann Surg. 275:591–5. DOI: 10.1097/SLA.0000000000004247. PMID: 32657945.
26. Achit H, Guillemin F, Karam G, Ladrière M, Baumann C, Frimat L, et al. 2020; Cost-effectiveness of four living-donor nephrectomy techniques from a hospital perspective. Nephrol Dial Transplant. 35:2004–12. DOI: 10.1093/ndt/gfz143. PMID: 31377771.
27. Arezzo A, Vettoretto N, Francis NK, Bonino MA, Curtis NJ, Amparore D, et al. 2019; The use of 3D laparoscopic imaging systems in surgery: EAES consensus development conference 2018. Surg Endosc. 33:3251–74. DOI: 10.1007/s00464-018-06612-x. PMID: 30515610.
28. Prudhomme T, Roumiguié M, Benoit T, Lesourd M, Beauval JB, Doumerc N, et al. 2019; Laparoscopy for living donor left nephrectomy: comparison of three-dimensional and two-dimensional vision. Clin Transplant. 33:e13745. DOI: 10.1111/ctr.13745. PMID: 31665808.
Fig. 1
The position of the surgeon’s hand and working trocars in hand-assisted laparoscopic donor nephrectomy.
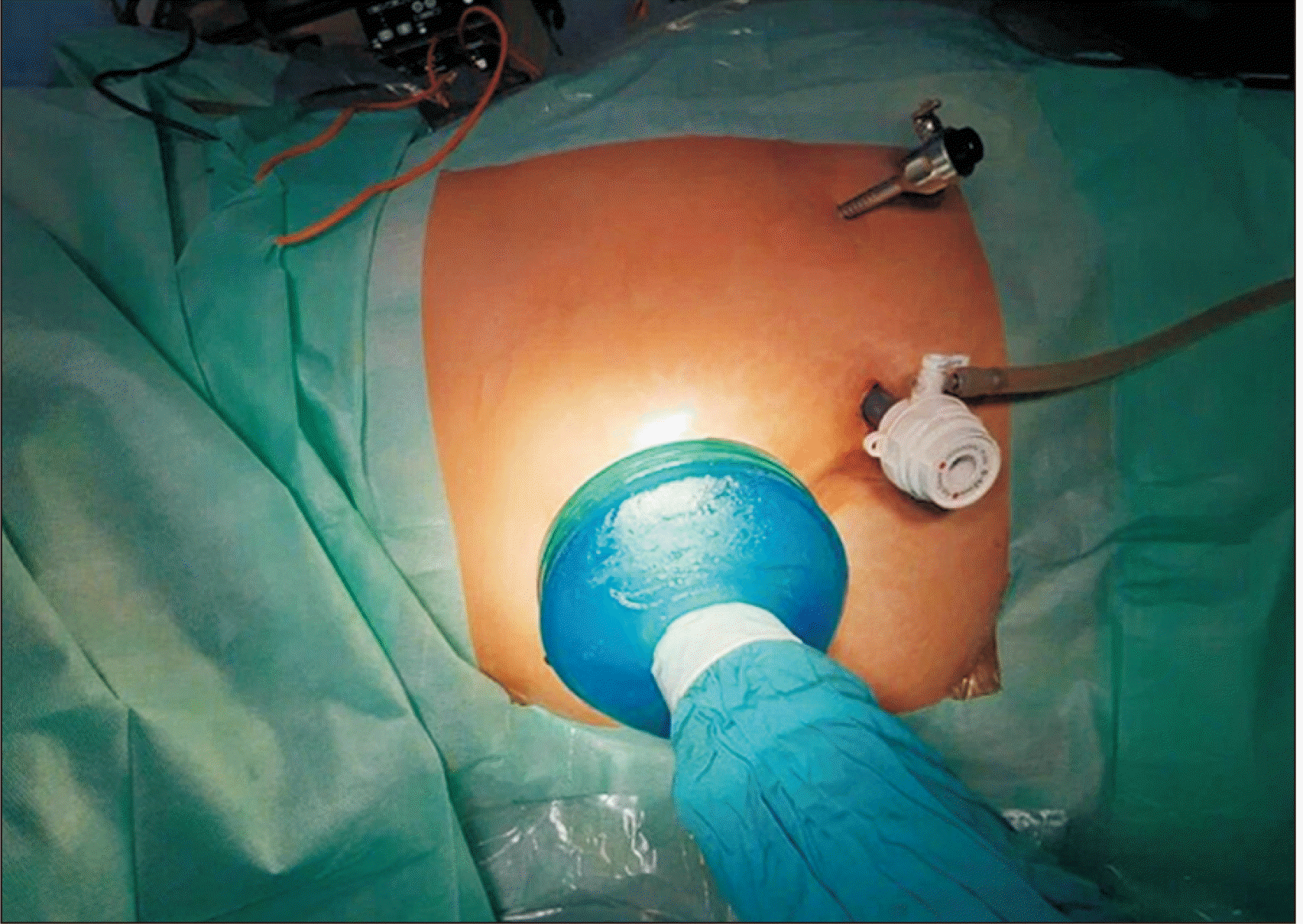
Table 1
Donors’ baseline characteristics
Table 2
Intraoperative data
Table 3
Postoperative outcomes




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download