INTRODUCTION
MATERIALS AND METHODS
Study population
Classification of carotid siphon
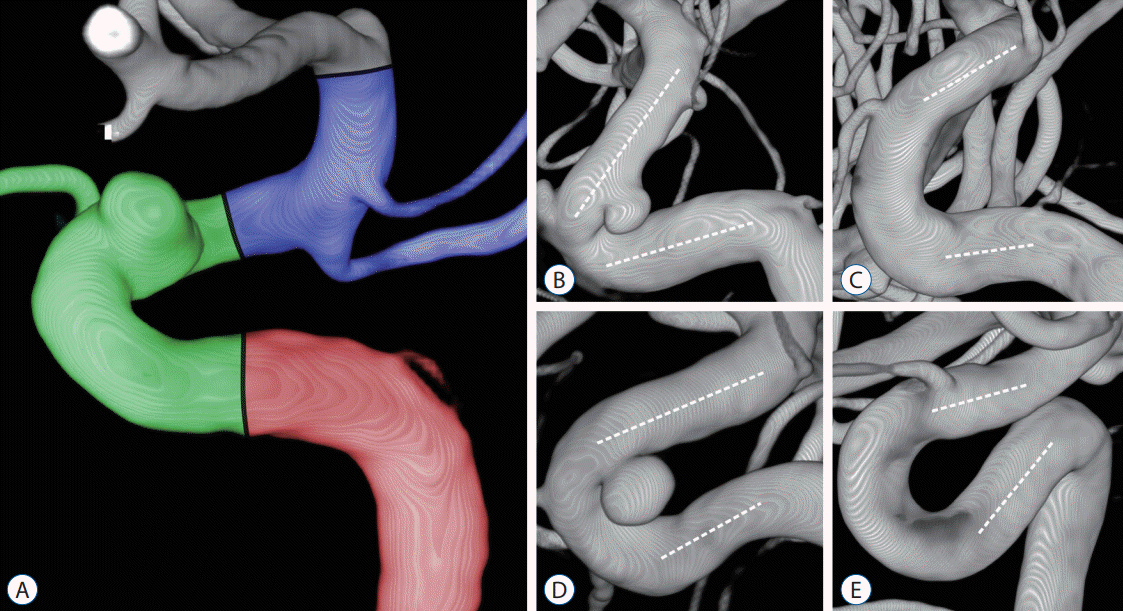 | Fig. 1.A : The carotid siphon was divided into posterior (red), anterior (green), and superior bends (blue). B-E : The anterior bends were categorized into angled (V) and non-angled (C, U, and S) types depending on morphology and measured angles. The angle of each bend was measured at the intersection between two lines traced through the midpoints of the diameters of each straight segment (white dotted lines). B : V-type. C : C-type (≥20°). D : U-type (≥-20°, <20°). E : S-type (<-20°). |
Antiplatelet regimen
Deployment of LVIS stent
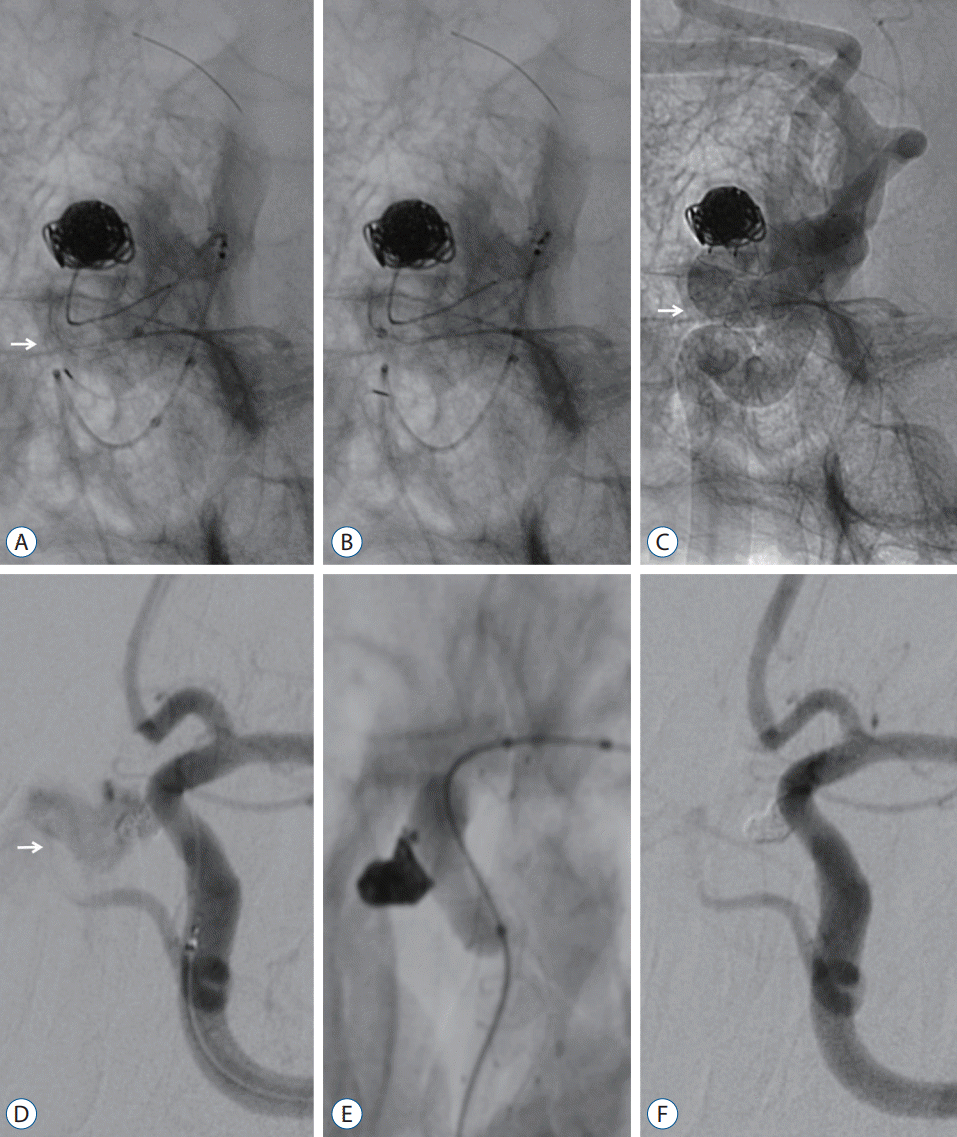 | Fig. 2.A-C : The push–pull technique was utilized when the two radiopaque strands within the stent body insufficiently contacted the vessel wall. A : Incomplete coverage at the anterior bend of the S-type (-25.5°) was identified during stent deployment (white arrow). B : The malpositioned stent was retrieved and redeployed while tension was provided by the push-back of the entire stent system. C : A fully expanded stent is shown on unsubtracted angiography (white arrow). D-F : Balloon angioplasty was only applied in cases of an intraoperative rupture of the aneurysm. D : Contrast leakage outside the lumen was observed due to the superior hypophyseal artery aneurysm rupture (white arrow). E : Balloon angioplasty was applied at the position of the aneurysm neck to stop the bleeding. F : There was no more hemorrhage, and the lesion was completely obliterated. |
Evaluation of stent apposition
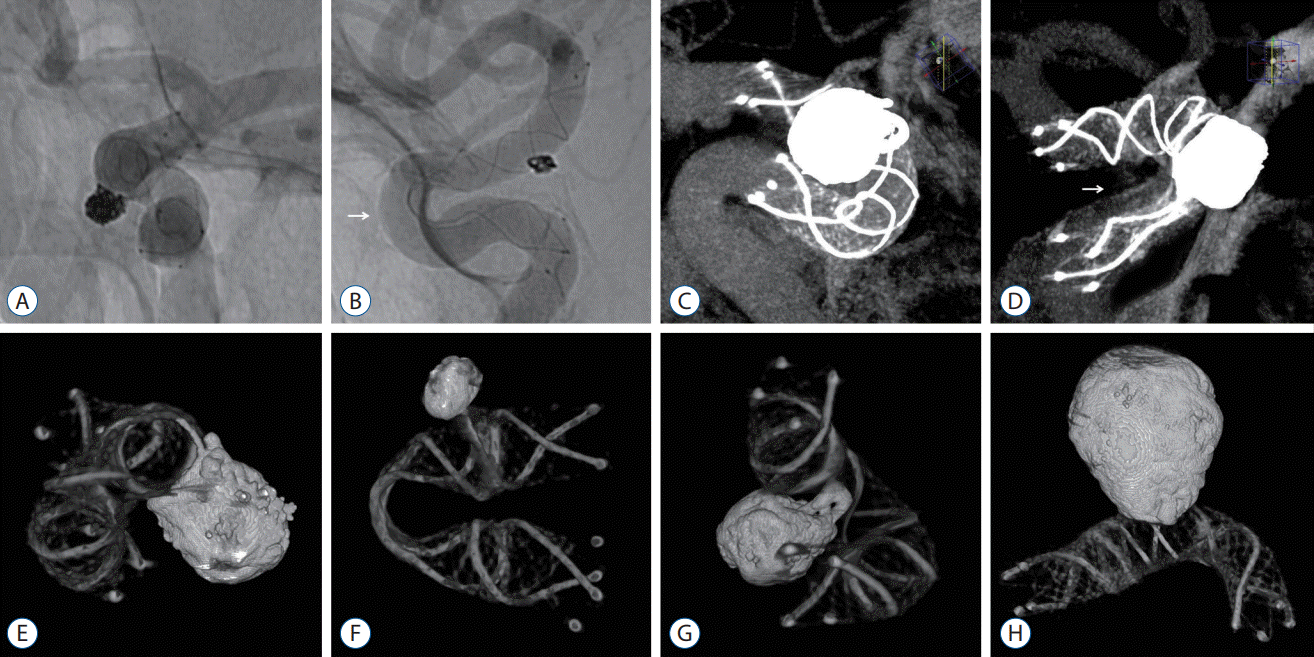 | Fig. 3.A : The status of stent apposition was evaluated by unsubtracted angiography, and the stent struts were in relatively good contact with the arterial wall. B : Trunk malposition was observed in the outer curvature of the anterior bend of the S-type (-45.8°) (white arrow). C : Flat-panel detector computed tomography (FDCT) with a contrast medium visualized the stent apposition through a multiplanar reconstruction in the maximal intensity projection mode. D : Trunk malposition was observed in the inner curvature of the anterior bend of the U-type (1.9°) (white arrow). E-G : FDCT without a contrast medium represented the metal components of the stent structure and coil mass as 3-dimensional reconstructed images and was taken to identify the degree of stent kinking and coil loop protrusion across the stent strut. H : The stent visualization was relatively well maintained, contrary to the concerns of metal artifacts arising from coil materials. |
Clinical and angiographic outcomes
Statistical analysis
RESULTS
Baseline characteristics
Table 1.
Values are presented as mean±standard deviation or number (%). LVIS : low-profile visualized intraluminal support, CSA : complete stent apposition, ISA : incomplete stent apposition, ICA : internal carotid artery, ARU : aspirin reaction unit, %INH : percentage inhibition, m/M : minimum-to-maximum, DAPT : dual antiplatelet therapy, SAPT : single antiplatelet therapy
ISA
IAEs and HAEs
Table 2.
| No. | Aneurysm location | Carotid siphon : anterior bend | Dyslipidemia | ARU | %INH | Stent apposition | Ischemic adverse events | Symptom | POD | Last | Aneury |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | OA | U | No | 461 | 0 | CSA | Intraoperative in-stent thrombosis | Asymptomatic | 0 | 0 | C/O |
| 2 | SHA | C | No | 407 | 7 | CSA | Intraoperative in-stent thrombosis | Asymptomatic | 0 | 0 | C/O |
| 3 | SHA | C | No | 555 | 10 | CSA | TIA | Visual disturbance | 2 | 0 | C/O |
| 4 | SHA | C | No | 392 | 7 | CSA | TIA | Visual disturbance | 9 | 0 | C/O |
| 5 | SHA | V | Yes | 511 | 6 | ISA | TIA | Visual disturbance | 16 | 0 | C/O |
| 6 | PDW | U | No | 405 | 0 | CSA | TIA | Visual disturbance | 26 | 0 | C/O |
| 7 | SHA | U | No | 432 | 0 | CSA | TIA | Photophobia | 10 | 0 | C/O |
| 8 | SHA | U | No | 426 | 23 | CSA | TIA | Photophobia | 12 | 0 | C/O |
| 9 | OA | V | Yes | 392 | 63 | ISA | TIA | Photophobia | 13 | 0 | Stabilization |
| 10 | SHA | C | No | 413 | 4 | CSA | TIA | Hypoesthesia | 1 | 0 | C/O |
| 11 | SHA | C | Yes | 398 | 45 | CSA | TIA | Hypoesthesia | 4 | 0 | C/O |
| 12 | OA | U | Yes | 427 | 20 | CSA | TIA | Hypoesthesia | 7 | 0 | C/O |
| 13 | PCoA | U | No | 439 | 5 | CSA | TIA | Hemiparesis | 8 | 0 | C/O |
| 14 | SHA | U | Yes | 487 | 0 | CSA | TIA | Hemiparesis | 23 | 0 | C/O |
| 15 | PCoA | C | Yes | 427 | 9 | CSA | TIA | Sensory aphasia | 1 | 0 | C/O |
| 16 | SHA | C | No | 398 | 0 | CSA | MCA territory infarction | Hemiplegia | 7 | 4 | Improvement |
| 17 | OA | U | Yes | 519 | 5 | CSA | MCA territory infarction* | Hemiplegia | 8 | 4 | C/O |
| 18 | SHA | S | Yes | 487 | 0 | ISA | MCA territory infarction | Hemiplegia | 86 | 0 | C/O |
| 19 | PDW | V | Yes | 567 | 21 | ISA | MCA territory infarction† | Hemiplegia | 169 | 0 | C/O |
| 20 | SHA | U | No | 413 | 22 | CSA | Thalamus infarction | Cognitive impairment | 4 | 2 | C/O |
| 21 | SHA | C | No | 379 | 23 | CSA | Delayed instent stenosis | Asymptomatic | 361 | 0 | C/O |
| 22 | OA | V | No | 376 | 15 | ISA | Delayed instent stenosis | Asymptomatic | 371 | 0 | C/O |
| 23 | OA | U | No | 473 | 28 | CSA | Delayed instent stenosis | Asymptomatic | 371 | 0 | C/O |
| 24 | OA | U | Yes | 409 | 20 | CSA | Delayed instent stenosis† | Asymptomatic | 375 | 0 | Improvement |
| 25 | PCoA | C | No | 401 | 5 | CSA | Delayed instent stenosis | Asymptomatic | 383 | 0 | Stabilization |
| 26 | SHA | C | No | 391 | 13 | CSA | Delayed instent stenosis | Asymptomatic | 391 | 0 | C/O |
| 27 | AchA | U | No | 481 | 21 | CSA | Delayed instent stenosis | Asymptomatic | 393 | 0 | C/O |
* The patient underwent mechanical thrombectomy to treat internal carotid artery occlusion due to the in-stent thrombosis.
LVIS : low-profile visualized intraluminal support, ARU : aspirin reaction unit, %INH : percentage inhibition, POD : postoperative day, mRS : modified Rankin Scale, OA : ophthalmic artery, CSA : complete stent apposition, C/O : complete occlusion, SHA : superior hypophyseal artery, TIA : transient ischemic attack, ISA : incomplete stent apposition, PDW : paraclinoid dorsal wall, PCoA : posterior communicating artery, MCA : middle cerebral artery, AchA : anterior choroidal artery
Immediate and follow-up degree of mRRC
Comparison of CSA and ISA
Predictive factors of ISA
Table 3.
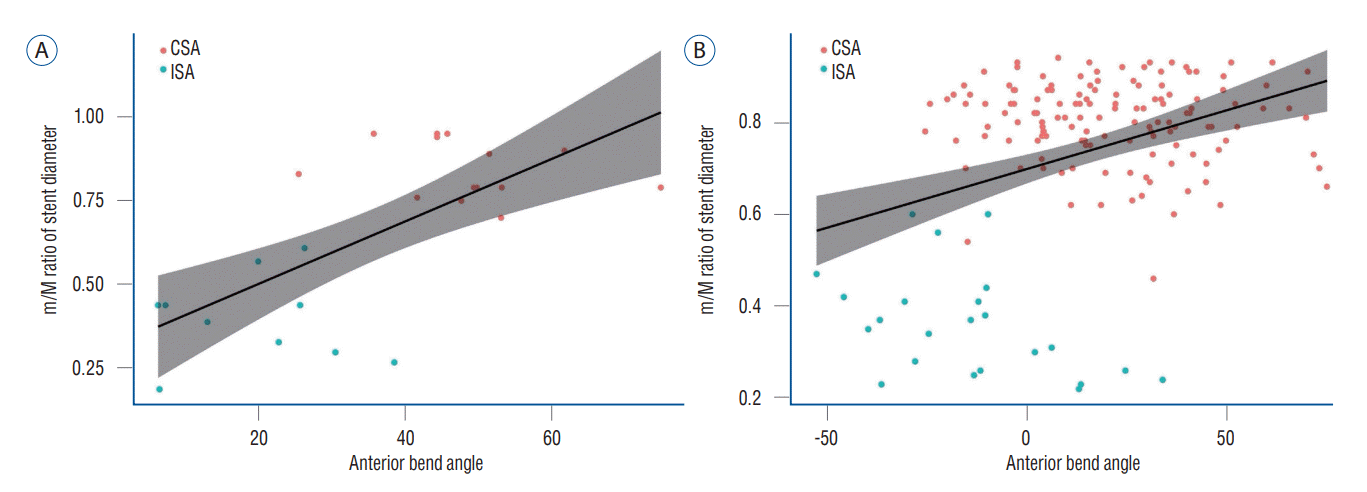 | Fig. 4.Pearson's correlation analysis showed a positive correlation between the anterior bend angle and the minimum-to-maximum ratio of stent diameter in both (A) angled (correlation coefficient, 0.69; p<0.001) and (B) non-angled types (correlation coefficient, 0.36; p<0.001). CSA : complete stent apposition, ISA : incomplete stent apposition. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download