Abstract
Objective
High-dose radiation is well known to induce and modulate the immune system. This study was performed to evaluate the correlation between clinical outcomes and changes in natural killer cell activity (NKA) after Gamma Knife Radiosurgery (GKS) in patients with brain cancer.
Methods
We performed an open-label, prospective, cross-sectional study of 38 patients who were treated with GKS for brain tumors, including metastatic and benign brain tumors. All of the patients underwent GKS, and blood samples were collected before and after GKS. NKA was measured using an enzyme-linked immunosorbent assay kit, to measure interferon-gamma (IFNγ) secreted by ex vivo-stimulated NK cells from whole blood. We explored the correlations between NK cell-produced IFNγ (NKA-IFNγ) levels and clinical parameters of patients who were treated with GKS for brain tumors.
Results
NKA-IFNγ levels were decreased in metastatic brain tumor patients compared to those with benign brain tumors (p<0.0001). All the patients who used steroid treatment to reduce brain swelling after GKS had an NKA-IFNγ level of zero except one patient. High NKA-IFNγ levels were not associated with a rapid decrease in brain metastasis and did not increase after GKS.
Radiotherapy (RT) for cancer treatment is well known to elicit an antitumor response by inducing the production of some cytokines [5,12,13]. Radiation can activate an interconnected network of several cytokines, which generates proinflammatory signaling [26]. This radiation-induced immune response is also suggested to occur as a result of high-dose irradiation of tumors. Preclinical studies have shown that high-dose irradiation can stimulate antitumor T cell immunity by promoting cross-priming by antigen-specific dendritic cells to increase the number of activated CD8+ T cells [9,11,16].
Of note, Gamma Knife Radiosurgery (GKS) for brain metastases have recently known to enhance the immune response against the tumor, even though brain as “immuneprivilege” [12]. Particularly, natural killer (NK) cells are known to play an important role in antitumor immunity, stimulated by radiation [6]. Activation of NK cells induce target cell apoptosis though cytotoxicity by perforin and granzyme B [7,24] and secretion of proinflammatory cytokines such as tumor necrosis factor-alpha and interferon-gamma (IFNγ) [4,37].
Recently, we measured the activity of NK cells by monitoring the level of IFNγ in serum using the NK Vue kit (NK MAX, Sungnam, Korea). This kit is an in vitro diagnostic test system that can measure NK cell activity (NKA)-IFNγ levels using enzymatic immunoassays. Previous studies have reported that compared to healthy subjects, patients with malignancy have significantly decreased levels of NKA-IFNγ [19,21]. Based on these findings, we investigated the activity of NK cells before and after GKS by evaluating the levels of IFNγ via the NK Vue kit. For evaluating the immunologic changes after GKS, we conducted prospectively preliminary research including brain metastatic patients as well as benign brain tumor patients, such as meningioma and schwannoma.
An independent Institutional Review Board of Seoul National University Hospital approved (H-1701-075-824).
This study was an open-label, prospective, cross-sectional clinical performance study for the measurement of NKA in whole blood (NK Vue; NK MAX) from subjects with a brain tumor. Subjects were enrolled between February and August 2018.
Patients were eligible for enrollment if they were male and older than 20 years old. Other eligibility criteria included providing informed consent for participation. Subjects could not participate if they could not understand the consent form or were unable to read it or if they had conditions shown to impact the activity of NK cells. The inclusion criteria were newly diagnosed brain tumor and no prior RT/radiosurgery within a minimum of the preceding three months. The exclusion criteria were high-grade glioma and a Karnofsky performance status lower than 70. The study also excluded patients who were receiving chemotherapy for a condition that could affect NK cell activity. Therefore, only patients who were not exposed to chemotherapy for at least one month were selected.
Blood sampling and NKA-IFNγ assay
All patients received a steroid injection (2 mg dexamethasone intravenously) to reduce peritumoral edema after GKS according to the protocol of the Gamma Knife Center. However, the steroid was only used just before and after GKS and was not used thereafter. Of the included patients, 10 patients had blood sampling performed before steroid injection and were used as a control group, and the remaining patients had sampling performed after a steroid injection and immediately before GKS. All patients completed follow-up magnetic resonance imaging of the brain between 1 and 3 months after GKS, and blood sampling was conducted simultaneously. Therefore, the patients had two blood samples drawn, one before and one after GKS, to measure the level of NKA-IFNγ. Of note, the steroid was not used at the time of the blood sampling conducted after GKS, and there were no effects of NKAIFNγ levels by pre-GKS steroid injection.
NKA-IFNγ levels were determined by enzymatic immunoassay using the NK Vue kit (NK MAX), as described previously [21]. This method stimulates whole blood (1 mL) with Promoca, and engineered recombinant cytokines that activate NK cells explicitly, and then measures IFNγ levels released from activated NK cells. After incubation for 20–24 hours at 37°C, the levels of IFNγ were measured with an ELISA. The detectable range was 0.1–4000 pg/mL, and the total imprecision of two levels of controls was less than 15% coefficients of variation. Unlike traditional methods for measuring of NK cell activity, this assay is simple and standardized. As it analyzes the distribution of lymphocytes in the IFNγ expressing subset, this assay has become applicable in the clinical setting as an estimate of NK cell activity. Therefore, it has been demonstrated in several papers that the activity of NK cells measured in this particular way is reliable [27,28].
Statistical analyses were performed using IBM SPSS version 23 (IBM SPSS, Armonk, NY, USA) and GraphPad Prism 5 (GraphPad Inc., La Jolla, CA, USA) was used to create figures. Categorical data were compared with the chi-square test or Fisher’s exact test, as appropriate. Cox regression analysis was performed to evaluate the hazard ratio and corresponding 95% confidence interval (CI). The correlation between the level of NKA-IFNγ and the reduction in tumor volume rate was evaluated by the Pearson correlation coefficient. All p-values were two-tailed. Statistical significance was considered at p<0.05.
This study included 28 patients with metastatic brain tumors and 10 patients with benign brain tumors, such as schwannomas and meningiomas. Patient characteristics are shown in Table 1. Of the 28 patients with brain metastasis, the most common primary tumor site was the lungs (67.9%). The mean target volume was 7.20 cm3 (range, 0.03–27.46), and the mean GKS dose was 22.94 Gy (range, 15–30). Eight patients underwent fractionated GKS, and 13 patients were previously irradiated via GKS. One patient underwent whole-brain RT before GKS, and two patients underwent craniotomy. Primary tumor control was observed in 20 patients, and systemic tumor control was found in 18 patients at the time of the study. This means that the disease burden of the primary tumor or systemic metastases does not progress.
Clinically, the tumor volume after GKS decreased significantly, and the levels of NKA-IFNγ increased (Table 2). However, no correlation between the two parameters was observed (Pearson sample correlation coefficient, 0.021; p=0.92). Changes from the initial tumor volume and levels of NKA-IFNγ also had no statistically significant correlations (p=0.41).
There were no significant differences in the changes in the levels of NKA-IFNγ up to 3 months after GKS (Table 3). Factors affecting the levels of NKA-IFNγ, the use of chemotherapy pre-GKS or post-GKS, and the presence of primary tumor control, systemic control, or several brain metastases were compared, but no statistically significant factors were identified.
The levels of NKA-IFNγ pre-GKS, post-GKS in brain metastasis patients, and post-GKS in benign brain tumor patients are shown in Fig. 1. The levels of NKA-IFNγ in the patients who were injected with steroids before GKS were zero in all except one patient. Interestingly, the IFNγ levels of the patients with malignant tumors, such as brain metastases, were significantly lower than those of the patients with benign brain tumors.
In the present study, we observed changes in serum IFNγ levels in brain tumor patients after GKS. Additionally, it was found that the IFNγ levels of the patients with malignant brain tumors represented as metastatic tumors were lower than those of the patients with benign brain tumors. The results showed that the NKA-IFNγ of patients with malignant brain tumors was more suppressed than that of benign tumor patients. These findings might imply that increasing the activity of NK cells may potentiate immunotherapy for malignant brain tumors.
Several studies have investigated the immune system in brain tumor patients, mainly in the context of malignant glioma. These studies suggested that brain tumor patients suffer from broad immunosuppression due to lymphopenia, decreased lymphocyte proliferation, increased cytotoxic deficiencies, reduced Major Histocompatibility Complex (MHC) class I expression on monocytes, and predominant anti-inflammatory T-helper 2-type cytokine production [10,30,33]. To overcome extensive immunosuppression, NK cells were studied as an immunotherapeutic target because of the direct cellkilling effect mediated by the innate immune response. NK cells possess the innate ability to detect transformed cells and are thus crucial to cancer immunosurveillance and antitumor immunity without the requirement of prior antigen exposure [8]. They perform cytotoxic activities through granzymeand perforin-mediated apoptosis or expression of death receptor ligands such as FasL and tumor necrosis factor (TNF)- related apoptosis-inducing ligand [38]. However, many tumors evade surveillance by NK cells, resulting in a skewed phenotype and impaired functionality during cancer progression. Therefore, increasing the activation of NK cells has become one of the strategies for brain tumors. Since Stevens et al. [35] showed that NK cells infiltrate the tumor site in gliomas, carcinoma metastases, and meningiomas, functional studies of the antitumor response mediated by NK cells have been attempted. Poli et al. [31] demonstrated that NK cells diminish tumor growth by recruiting macrophages and inducing the overexpression of MHC class II on microglia as well as the elevation of IFNγ and TNF-α levels in the cerebrospinal fluid. Lee et al. [22] also showed that NK cell-mediated immunity is responsible for the suppression of systemic glioblastoma metastasis in mice. However, despite this evidence, the complex nature of the immune system makes NK cell-based therapies far from clinically successful strategies for brain tumor treatment.
Recently, there has been increasing evidence that localized irradiation of a tumor may also modify the tumor microenvironment and generate proinflammatory cytokines [25,26,32,40], which can increase the robustness of the immune response. The effect of radiation is known to be dependent on T cells, and their ability to recognize tumor antigens, primarily via RT-mediated T cell priming, contributes to the activation of host immunity. Even if high-dose irradiation, such as that applied with GKS, is considered and the RT differs in radiobiological aspects, there is ample evidence that radiosurgery also triggers an immune response against tumors [34,39]. Not only is GKS effective with programmed cell death 1 receptor antagonists for brain metastasis [1,29], but more recent evidence has shown that GKS regulates the immune response [12]. This report suggested that GKS for metastatic brain tumor patients are regulated cytokines such as IFNγ and interleukin-2 [12]. The results showed that the level of IFNγ at 1 hour post-GKS in metastatic brain tumor patients increased and that after 1 week was lower than that at 1 hour, but still increased before GKS. However, our results were discrepant, as IFNγ levels declined after GKS. In aprevious study, IFNγ levels decreased over time [12], and our findings were measured at least 1 month after GKS. These discrepancies are considered to need to be investigated later with more subjects and immune profiles. Overall, the IFNγ level after GKS is increased for at least 1 week, but it could be decreased when 1 month has passed. Interestingly, the IFNγ level after GKS for benign tumor patients was higher than that for metastatic brain tumor patients, representing the immunosuppressive status of metastatic brain tumor patients. Therefore, it is likely that improving the immunosuppressive status of metastatic brain tumor patients can be a basis for treatment.
Moreover, no correlations between the interval after GKS or the rate of reduction in tumor volume and changes in serum IFNγ levels were found. This means that the more IFNγ levels was measured, the less the size of tumor volume was reduced. This lack of correlation was probably because this study is based on prospective clinical data with a relatively small number of patients. Although our results did not show that GKS stimulates the immune response, it has been demonstrated in the literature that GKS stimulates a cellular immune response involving NK cells against brain metastases [36]. A previous study performed histopathological and immunohistochemical evaluations of 11 tumor tissue samples obtained by craniotomy from patients who received GKS. However, this study only produced cytological observations, demonstrating GKSreactive cells but not showing changes in the immune response. On the other hand, our results provided evidence that it could cause changes in the immune system after GKS. These findings have consistency with another previously mentioned study [12].
In this study, we measured IFNγ released from stimulated NK cells in 1 mL of fresh whole blood. This modern method of measuring NK cell activity is standardized and straightforward compared to flow cytometric assays [27]. This test has already been verified, and it has been used to investigate possible prognostic markers [2,14,15,20]. However, no studies have been performed to determine the activity of NK cells after GKS in brain tumor patients. Interestingly, the levels of NKA-IFNγ in patients treated with steroids were low. Considering that steroids inhibit NK cell activity, this test might be reliable.
Recent studies have shown that the systemic immune status does not differ from the brain’s immune status. The traditional concept that the blood-brain barrier makes the brain immune system different from the systemic immune system has been completely changed due to the discovery of brain lymphatic vessels by Kipnis and colleagues [18,23]. In parallel with these discoveries, recent studies have demonstrated the presence of microglia and other immune cells within the brain that have been shown to respond to systemic cytokines [3,17,18]. These findings provide supporting evidence that the brain is not as ‘immunoprivileged’ as previously accepted. These findings imply that the systemic immune status reflects the brain’s immune response. In other words, serum levels of NKA-IFNγ could indicate the brain’s immune status. Serum levels of NKA-IFNγ could also be used as a therapeutic or diagnostic marker as they are significantly decreased in malignant brain tumors relative to benign brain tumors. Additionally, we expect that it will serve as a basis for use as a predictive marker in reducing the clinical confusion that occurs after GKS, such as radiation-necrosis, pseudo-progression, and recurrence.
This study has several limitations because of its relatively small sample size and lack of evaluation of other immunological factors, such as interleukins, chemokines, and tumor necrosis factor. The heterogeneity of the small sample size was the reason why the unclear results become. The most significant limitation is that there are no additional cytokine data to comprehensively evaluate the post-GKS immune system for brain tumors. Moreover, our study has a crucial limitation as no consecutive data. Because only the pre- and pos-GKS NKA-IFNγ were measured without baseline NKA-IFNγ, there is a potent confusion about the immune effects of GKS. Additionally, even if IFNγ is a potent immunomodulator and reflects the activity of NK cells, questions remain as to whether the levels of NKA-IFNγ can represent the immune status of brain tumor patients. Finally, our results did not provide the level of NKA-IFNγ in healthy people. Although previous studies have identified the values of the normal range [2,14,15,20], the incomplete results make relative comparisons difficult and potentially confusing.
Despite these limitations, our study has the novelty of being a valuable investigation to evaluate the immune response in brain tumor patients after GKS. Because the levels of NKAIFNγ after GKS were significantly lower in malignant tumors than in benign tumors, NKA-IFNγ might have diagnostic value as a prognostic marker. Additionally, these results indicate that evaluating the activation of NK cells is a promising strategy for estimating the immune status of patients with brain tumors as well as their immune conditions before and after brain tumor treatment. Although further clinical studies with larger populations are required to confirm the immunological changes after GKS in patients with brain tumors, our results suggest that GKS might modulate the immune response in patients with brain tumors.
Notes
Informed consent
Informed consent was obtained from all individual participants included in this study.
References
1. Ahn MJ, Lee K, Lee KH, Kim JW, Kim IY, Bae WK. Combination of antiPD-1 therapy and stereotactic radiosurgery for a gastric cancer patient with brain metastasis: a case report. BMC Cancer. 18:173. 2018.
2. Barkin J, Rodriguez-Suarez R, Betito K. Association between natural killer cell activity and prostate cancer: a pilot study. Can J Uro. 24:8708–8713. 2017.
3. Berghoff AS, Preusser M. The inflammatory microenvironment in brain metastases: potential treatment target? Chin Clin Oncol. 4:21. 2015.
4. Caligiuri MA. Human natural killer cells. Blood. 112:461–469. 2008.
5. Carvalho HA, Villar RC. Radiotherapy and immune response: the systemic effects of a local treatment. Clinics (Sao Paulo). 73(suppl 1):e557s. 2018.
6. Chen J, Liu X, Zeng Z, Li J, Luo Y, Sun W, et al. Immunomodulation of NK cells by ionizing radiation. Front Oncol. 10:874. 2020.
7. Cho D, Campana D. Expansion and activation of natural killer cells for cancer immunotherapy. Korean J Lab Med. 29:89–96. 2009.
8. Dahlberg CI, Sarhan D, Chrobok M, Duru AD, Alici E. Natural killer cellbased therapies targeting cancer: possible strategies to gain and sustain anti-tumor activity. Front Immunol. 6:605. 2015.
9. Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 15:5379–5388. 2009.
10. Dix AR, Brooks WH, Roszman TL, Morford LA. Immune defects observed in patients with primary malignant brain tumors. J Neuroimmunol. 100:216–232. 1999.
11. Frey B, Rubner Y, Kulzer L, Werthmöller N, Weiss EM, Fietkau R, et al. Antitumor immune responses induced by ionizing irradiation and further immune stimulation. Cancer Immunol Immunother. 63:29–36. 2014.
12. Hatiboglu MA, Kocyigit A, Guler EM, Nalli A, Akdur K, Sakarcan A, et al. Gamma knife radiosurgery compared to whole brain radiation therapy enhances immunity via immunoregulatory molecules in patients with metastatic brain tumours. Br J Neurosurg. 34:604–610. 2020.
13. Jeong H, Bok S, Hong BJ, Choi HS, Ahn GO. Radiation-induced immune responses: mechanisms and therapeutic perspectives. Blood Res. 51:157–163. 2016.
14. Jobin G, Rodriguez-Suarez R, Betito K. Association between natural killer cell activity and colorectal cancer in high-risk subjects undergoing colonoscopy. Gastroenterology. 153:980–987. 2017.
15. Jung YS, Kwon MJ, Park DI, Sohn CI, Park JH. Association between natural killer cell activity and the risk of colorectal neoplasia. J Gastroenterol Hepatol. 33:831–836. 2018.
16. Kaur P, Asea A. Radiation-induced effects and the immune system in cancer. Front Oncol. 2:191. 2012.
17. Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, DvirSzternfeld R, Ulland TK, et al. A unique Microglia type associated with restricting development of alzheimer’s disease. Cell. 169:1276–1290.e17. 2017.
18. Kipnis J, Filiano AJ. Neuroimmunology in 2017: the central nervous system: privileged by immune connections. Nat Rev Immunol. 18:83–84. 2018.
19. Koo KC, Shim DH, Yang CM, Lee SB, Kim SM, Shin TY, et al. Reduction of the CD16(-)CD56bright NK cell subset precedes NK cell dysfunction in prostate cancer. PLoS One. 8:e78049. 2013.
20. Lee J, Park KH, Ryu JH, Bae HJ, Choi A, Lee H, et al. Natural killer cell activity for IFN-gamma production as a supportive diagnostic marker for gastric cancer. Oncotarget. 8:70431–70440. 2017.
21. Lee SB, Cha J, Kim IK, Yoon JC, Lee HJ, Park SW, et al. A high-throughput assay of NK cell activity in whole blood and its clinical application. Biochem Biophys Res Commun. 445:584–590. 2014.
22. Lee SJ, Kang WY, Yoon Y, Jin JY, Song HJ, Her JH, et al. Natural killer (NK) cells inhibit systemic metastasis of glioblastoma cells and have therapeutic effects against glioblastomas in the brain. BMC Cancer. 15:1011. 2015.
23. Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 523:337–341. 2015.
24. Luetke-Eversloh M, Killig M, Romagnani C. Signatures of human NK cell development and terminal differentiation. Front Immunol. 4:499. 2013.
25. McBride WH, Chiang CS, Olson JL, Wang CC, Hong JH, Pajonk F, et al. A sense of danger from radiation. Radiat Res. 162:1–19. 2004.
26. McKelvey KJ, Hudson AL, Back M, Eade T, Diakos CI. Radiation, inflammation and the immune response in cancer. Mamm Genome. 29:843–865. 2018.
27. Nederby L, Hansen T, Raunkilde L, Jensen LH, Jakobsen AKM. Natural killer cell activity: a test for immune reactivity with clinical perspectives. JCO. 36 5_suppl:87–2018.
28. Nederby L, Jakobsen A, Hokland M, Hansen TF. Quantification of NK cell activity using whole blood: methodological aspects of a new test. J Immunol Methods. 458:21–25. 2018.
29. Nordmann N, Hubbard M, Nordmann T, Sperduto PW, Clark HB, Hunt MA. Effect of gamma knife radiosurgery and programmed cell death 1 receptor antagonists on metastatic melanoma. Cureus. 9:e1943. 2017.
30. Parney IF. Basic concepts in glioma immunology. Adv Exp Med Biol. 746:42–52. 2012.
31. Poli A, Wang J, Domingues O, Planagumà J, Yan T, Rygh CB, et al. Targeting glioblastoma with NK cells and mAb against NG2/CSPG4 prolongs animal survival. Oncotarget. 4:1527–1546. 2013.
32. Schoenhals JE, Seyedin SN, Anderson C, Brooks ED, Li YR, Younes AI, et al. Uncovering the immune tumor microenvironment in non-small cell lung cancer to understand response rates to checkpoint blockade and radiation. Transl Lung Cancer Res. 6:148–158. 2017.
33. Servadei F, Parente R, Bucci M, Beltrandi E, Tognetti F, Gaist G. Particular features of cell-mediated immunity in patients with anaplastic gliomas. A comparison with kidney and bladder cancer patients. J Neurooncol. 1:327–332. 1983.
34. Sologuren I, Rodríguez-Gallego C, Lara PC. Immune effects of high dose radiation treatment: implications of ionizing radiation on the development of bystander and abscopal effects. Transl Canc Res. 3:18–31. 2014.
35. Stevens A, Klöter I, Roggendorf W. Inflammatory infiltrates and natural killer cell presence in human brain tumors. Cancer. 61:738–743. 1988.
36. Szeifert GT, Salmon I, Rorive S, Massager N, Devriendt D, Simon S, et al. Does gamma knife surgery stimulate cellular immune response to metastatic brain tumors? A histopathological and immunohistochemical study. J Neurosurg. 102 Suppl:180–184. 2005.
37. Vivier E, Ugolini S. Natural killer cells: from basic research to treatments. Front Immunol. 2:18. 2011.
38. Wallin RP, Screpanti V, Michaëlsson J, Grandien A, Ljunggren HG. Regulation of perforin-independent NK cell-mediated cytotoxicity. Eur J Immunol. 33:2727–2735. 2003.
39. Zhang T, Yu H, Ni C, Zhang T, Liu L, Lv Q, et al. Hypofractionated stereotactic radiation therapy activates the peripheral immune response in operable stage I non-small-cell lung cancer. Sci Rep. 7:4866. 2017.
40. Zhi-Feng W, Le-Yuan Z, Xiao-Hui Z, Ya-Bo G, Jian-Ying Z, Yong H, et al. TLR4-dependent immune response promotes radiation-induced liver disease by changing the liver tissue interstitial microenvironment during liver cancer radiotherapy. Radiat Res. 182:674–682. 2014.
Fig. 1.
Comparison of natural killer cell activities pre- and post-Gamma Knife Radiosurgery (GKS). *p<0.01 vs. the control group. †p<0.001 vs. the control group. NKA-IFNγ : natural killer cell-produced interferon-gamma.
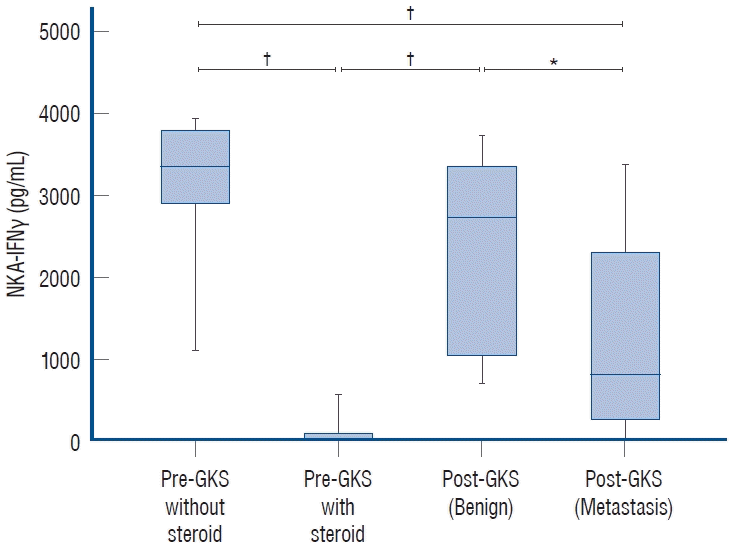
Table 1.
Patient characteristics (38 patients)
| Value | |
|---|---|
| Age (years) | 52.6 (26–77) |
| Sex, male/female | 12/26 |
| Primary tumor site | |
| Lung | 19 (67.9) |
| Breast | 4 (14.3) |
| Etc.* | 5 (17.8) |
| Benign | |
| Meningioma | 7 (70.0) |
| Schwannoma | 3 (30.0) |
| Mean target volume (cm3) | 7.20 (0.03–27.46) |
| No. of brain metastases | 3.57 (1–10) |
| Total GKS dose (Gy) | 22.94 (15–30) |
| Fractionated GKS | 8 (21.1) |
| Previous GKS treatments | 13 (32.2) |
| 1 more | 10 |
| 2 more | 1 |
| 3 more | 1 |
| 4 more | 1 |
| Previous WBRT | 1 (2.6) |
| Previous craniotomy | 2 (5.3) |
| Primary control patients† | 20 (52.6) |
| Systemic control patients† | 18 (47.3) |
Table 2.
Clinical outcomes of patients with brain metastasis
| Pre-GKS | Post-GKS | p-value | |
|---|---|---|---|
| Tumor volume (cm3) | 7.20±7.82 | 4.95±5.52 | 0.01 |
| WBC count (1000/mL) | 6.84±3.21 | 5.84±1.99 | N/S |
| IFNγ level (pg/mL) | 59.41±150.58 | 1198.25±1151.79 | <0.001 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download