Abstract
The Kernohan-Woltman notch phenomenon (KWNP) refers to an intracranial lesion causing massive side-to-side mass effect which leads to compression of the contralateral cerebral peduncle against the free edge of the cerebellar tentorium. Diagnosis is based on “paradoxical” motor deficit ipsilateral to the lesion associated with radiologic evidence of damage to the contralateral cerebral peduncle. To date, there is scarce evidence regarding KWNP associated neuroimaging patterns and motor function prognostic factors. A systematic review was conducted on Medline database from inception to July 2021 looking for English-language articles concerning KWNP, in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The research yielded 45 articles for a total of 51 patients. The mean age was 40.7 years-old and the male/female sex ratio was 2/1. 63% of the patients (32/51) suffered from head trauma with a majority of acute subdural hematomas (57%, 29/51). 57% (29/51) of the patients were in the coma upon admission and 47% (24/51) presented pupil anomalies. KWNP presented the neuroimaging features of compression ischemic stroke located in the contralateral cerebral peduncle, with edema in the surrounding structures and sometimes compression stroke of the cerebral arteries passing nearby. 45% of the patients (23/51) presented a good motor functional outcome; nevertheless, no predisposing factor was identified. A Glasgow coma scale (GCS) of more than 3 showed a trend (p=0.1065) toward a better motor functional outcome. The KWNP is a regional compression syndrome oftentimes caused by sudden and massive uncal herniation and leading to contralateral cerebral peduncle ischemia. Even though patients suffering from KWNP usually present a good overall recovery, patients with a GCS of 3 may present a worse motor functional outcome. In order to better understand this syndrome, future studies will have to focus on more personalized criteria such as individual variation of tentorial notch width.
A few intracranial lesions such as an acute subdural hematoma (ASH) or a cerebral tumor with major cerebral edema can cause important side-to-side mass effect, eventually leading to uncal herniation with ipsilateral mydriasis and contralateral hemiparesis. Such level of preoperative compression of the adjacent cerebral parenchyma may be responsible for long-term neurologic impairment including contralateral motor deficit. The decision whether to perform surgery in the acute setting in comatose patients can be challenging given that even the patient awakening remains uncertain [16]. In extreme situations, it occurs that the side-to-side mass effect on the temporal lobe causes temporal uncus herniation with major brainstem displacement and finally compression of the contralateral cerebral peduncle against the free edge of the cerebellar tentorium. During such phenomenon, patients present a “paradoxical” motor deficit ipsilateral to the side of the lesion because of damage to the contralateral corticospinal tract (CST) passing through the cerebral peduncle. This peculiar type of uncal herniation has been described for the first time in autopsic series for brain tumors in 1927 by Groeneveld and Schaltenbrand [22] and then in 1929 by Kernohan and Woltman [31]. To date, it has been essentially reported through case reports and case series. Consequently, there is a lack of evidence regarding the functional outcome of these patients who often present a serious neurological condition upon admission, but generally overcome this overwhelming neurologic event with a rather good Glasgow outcome scale (GOS) score. The aim of this study is to identify the potential factors influencing the middle-term and long-term motor functional outcome of patients suffering from Kernohan-Woltman notch phenomenon (KWNP) in order to assist neurosurgeons in the surgical decision making and the rehabilitation orientation. To this end, we also try to better understand the pathophysiology of KWNP with a precise study of neuroimaging features.
We conducted a systematic literature review focused on KWNP on Medline database (https://pubmed.ncbi.nlm.nih.gov/) from inception until July 2021. We used the advanced research mode with the following MeSH terms : “Kernohan” in the title or “Kernohan” in the text.
All the English-language articles with individual extractable data concerning a case of KWNP were included in the quantitative analysis. The exclusion criteria were article not written in English, articles not directly relevant to the subject, article that could not be found despite being indexed in MEDLINE, and articles with no individual extractable data.
All the articles included in the quantitative analysis were screened in a systematic manner and the following information was extracted as previously planned : author and year of publication, age and sex of the patient, relevant comorbidities, mechanism and etiology of the responsible disease, neurological examination upon admission including the Glasgow coma scale (GCS), pupils, and focal symptoms, findings on the initial neuroimaging, surgery or intervention, immediate post-operative neurological examination including pupils and focal symptoms, diagnostic morphologic imaging, diagnostic neurophysiological tests including motor evoked potentials or somatosensory evoked potentials, long-term neurological sequelae, and delay of the last follow-up. This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [38].
The primary endpoint of the study was to assess the potential factors influencing the middle-term and long-term motor functional outcome of the patients. We defined a good outcome when there was no motor deficit, or when motor function was reported as “mild hemiparesis”, “slight hemiparesis” or ranked at least three out of five on the modified Medical Research Council (mMRC) scale [11] in the case reports. In the contrary, a bad functional outcome was defined when the motor function was reported as “hemiparesis”, “severe hemiparesis” or when it was ranked lower than three out of five on the mMRC scale, when the patient suffered from hemi-parkinsonism, or when death occurred. The secondary endpoints included the description of the main etiologies causing KWNP, and the analysis of the magnetic resonance imaging (MRI) and any other neurophysiological tests available in order to provide a better understanding of KWNP pathophysiology.
All statistical analyses were conducted using R software version 4.1.0 (R Core Team, Vienna, Austria). Categorical variables were presented as numbers and percentages, and continuous variables were presented as median and interquartile range. The motor functional prognosis was defined as positive or bad using the above-mentioned criteria. Differences in the functional prognosis depending on clinical data (age, GCS, and pupillary status upon admission), radiologic findings (epidural hematoma [EDH], ASH, and chronic subdural hematoma [CSH]) and surgical procedure (craniotomy or craniectomy) were assessed with univariate analysis using Fisher’s exact test for categorical variables given the small size of this series. A two-sided p-value of less than 0.05 was considered to indicate statistical significance.
Reports concerning patients suffering from KWNP ranged from 1990 to 2020 [2-10,13-15,17,19-21,23,26-30,33-37,39-41,43-46,49,50,52-55,59-63]. The database research yielded 220 articles and 149 after removal of duplicates. After the first screening, two articles were excluded because they were not found and four articles because they were written in foreign language. Among the 143 articles retained, 98 also met the exclusion criteria after full reading of the content. At last, 45 articles, including 39 case reports and six case series (two to three patients), were retained in the quantitative analysis for a total of 51 patients (Supplementary Table 1). PRISMA flow diagram is provided in Fig. 1. The mean age of the patients in this review was 40.7 (±20.8) years old and the sex-ratio was two males for one female patient.
The majority of the patients (32/51, 63%) were admitted after head trauma, whether it was caused by a blunt instrument or an aggression (9/51, 18%), a fall (7/51, 13.7%), a car crash (6/51, 12%) or a motorcycle accident (1/51, 2%), or an unknown mechanism (9/51, 18%). Of note, two cases (4%) of young men aged 19 and 29 years old were admitted for coma soon after boxing. Sixteen patients (39%) were admitted for a spontaneous neurological event. Two patients (4%) had a recent history of neurosurgical intervention. Finally, one patient (2%) benefited from a depletive lumbar puncture a few days before (Table 1).
Forty-eight patients (94%) were admitted for acute-onset symptoms and three patients (6%) were admitted with a chronic case history. More than half of the patients (29/51, 57%) presented the clinical criteria of coma with a GCS score of eight or less upon admission. Notably, eight patients (16%) presented a GCS of three. Twelve patients (24%) presented a GCS higher than eight, among whom one third suffered from CSH. The level of awareness was not mentioned for 10 patients (20%).
Pupil anomalies were reported in 24 patients (47%), with a majority (19/51, 37%) of ipsilateral mydriasis. Contralateral mydriasis was reported in three patients (6%) and there were two cases (4%) with bilateral mydriasis. Four patients (8%) presented with ipsilateral oculomotor palsy. The pupil examination was not mentioned for 23 patients (45%).
Ipsilateral motor deficit was reported in 15 patients (29%), with 12 cases (24%) of ipsilateral hemiparesis and three cases (6%) of ipsilateral hemiplegia. Two patients (4%) with slowly progressing disease (an arachnoid cyst and a diffuse glioma) presented contralateral hemiparesis, in which case the diagnosis of KWNP was based upon radiologic features. One patient (2%) with malignant ischemic stroke presented contralateral hemiplegia. Motor function was not reported in 33 patients (65%) (Table 2).
Concerning the patients with a chronic case history, one presented a chronic hemiparesis revealing a CSH, the second one presented a 2-years history of facial palsy and falls revealing an arachnoid cyst, and the last one suffered from a one-year history of cognitive impairment, falls and hemiparesis caused by a diffuse glioma.
Forty-six patients (90%) benefited from a computed tomography (CT) scan of the head upon admission. The majority (42/51, 82%) of the patients suffered from brain trauma with 29 cases (57%) of extra-axial hematomas and two cases (4%) of contusions or diffuse axonal injury. Twenty-nine patients (57%) suffered from ASH, three of which were caused by an arteriovenous malformation (AVM) and one by an aneurysm. Six patients (12%) presented CSH and seven (14%) presented an EDH. Last, subarachnoid hemorrhage from ruptured aneurysm, an arachnoid cyst, multiloculated hydrocephalus, depressed temporal skull fracture, an intracerebral hemorrhage, malignant ischemic stroke, and diffuse glioma were encountered once each. Among the vascular lesions, the two ruptured aneurysms were diagnosed using CT scan with angiography solely, whereas two out of three cases of ruptured AVM benefited from urgent digital subtraction angiography. Four patients (8%) benefited from a MRI of the brain upon admission, which revealed an arachnoid cyst, a CSH, a high-grade glioma, and a case of multiloculated hydrocephalus. One patient (2%) benefited from both a CT scan and a brain MRI which revealed a CSH. The urgent neuroimaging modality was missing in one case of head trauma, in which case MRI of the brain during hospitalization revealed diffuse axonal injury (Table 3).
Forty-eight patients (94%) were operated on in an emergency setting. Thirty-four patients (67%) benefited from craniotomy, including trephination and burr-hole for CSH or placement of an external ventricular drain, whereas 14 patients (27%) benefited from decompressive craniectomy (DC). Only two cases of DC were carried out until 2010 which represents nine percent of all neurosurgical procedures during this period. At the opposite, 12 patients (48%) benefited from DC since 2013. DC was most of the time performed for ASH; in fact, 10 patients (34%) with ASH benefited from DC whereas 19 patients (66%) benefited from craniotomy. Otherwise, DC was carried out for a case of bilateral EDH and contusions, for a malignant ischemic stroke, for a CSH, and for a Fisher 4 subarachnoid hemorrhage. One case (2%) of ASH and one case (2%) of depressed temporal skull fracture were managed with an external ventricular drain solely.
Regarding the vascular malformations, two out of three AVM were treated in an emergency setting : one was operated on and the other one was embolized before being operated on. The third one was embolized in a delayed fashion after that the patient has recovered properly. The two cerebral aneurysms encountered in this review were treated in an emergency setting : one benefited from neurosurgical clipping in 1992 and the other one from endovascular coiling in 2017.
One case of sylvian arachnoid cyst was marsupialized using craniotomy. Finally, one case of multiloculated hydrocephalus benefited from extensive fenestration of intraventricular septa using craniotomy and was re-admitted soon afterwards for acute hydrocephalus, which required ventriculoperitoneal shunting after a short period of external ventricular drainage.
The immediate post-operative neurological status was reported in 27 patients (53%). Regarding the pupil examination, four patients (8%) presented ipsilateral cranial nerve (CN) III deficit in the early post-operative period, among which three cases were noticed pre-operatively. One patient (2%) presented a contralateral CN III deficit which was noticed before the procedure. Four patients (8%) with pre-operative ipsilateral mydriasis presented a normal pupil examination afterwards. The pupil examination was not reported in 42 patients (82%). Regarding the motor examination, 15 patients (29%) presented ipsilateral hemiparesis and nine patients (18%) presented ipsilateral hemiplegia. One case (2%) of CSH with pre-operative ipsilateral mydriasis and hemiparesis fully recovered after the procedure. One patient (2%) with ASH who benefited from DC presented ipsilateral CN III deficit, ipsilateral hemiparesis and ipsilateral quadranopsia after the procedure. One patient with Fisher four subarachnoid hemorrhage presented a locked-in syndrome after the procedure. The motor examination was not reported in 25 cases (49%).
Forty-seven patients (92%) benefited from MRI of the brain. Among them, the cerebral peduncle contralateral to the lesion was pathological in 44 patients (86%) and normal in three patients (6%). In a subacute setting, the lesion in the cerebral peduncle presented hypointense signal in T1-weighted sequence [28,36,40,41,50,62] and hyperintense signal in T2-weighted sequ ence [2,3,5,10,13,20,21,23,28,33,34,36,37,39-41,46,50,52-55,61,63] and in fluid-attenuated inversion recovery (FLAIR) sequence [2,4,14,19-21,34,37,60]. It displayed restricted diffusion with brightness in diffusion-weighted imaging (DWI) [7,9,14,21,34,39,52-54], and apparent diffusion coefficient low values [9,54]. Last, it presented hypointense signal in gradient echo sequence [5] and evidence of blood-brain barrier rupture in post-contrast T1-weighted imaging [21]. Middle-term and long-term aspect displayed hypointense signal in T1-weighted sequence [27], and hyperintense signal in T2-weighted [8,15,27,44] and FLAIR imaging [44].
In case where the cerebral peduncle was considered as normal on brain MRI, diffusion tensor imaging with tractography was often carried out and displayed a narrowing or an interruption in the CST contralateral to the lesion [28,29,35,61]. What is more, contralateral structures surrounding the contralateral cerebral peduncle such as the thalamus [41] and internal capsule [2,19,21,37], the substantia nigra [15], the splenium of the corpus callosum [60] and the occipital lobe [41,60] presented signal anomalies. Last, a few cases presented evidence of acute ischemic event in the territory of the superior cerebellar artery (SCA) contralateral to the lesion [34,39] and the posterior cerebral artery (PCA) ipsilateral to the lesion [34] on DWI (Table 3 and Fig. 2).
Transcranial evoked potentials were recorded for scientific purpose [2,61], in case patients were unresponsive [21], or for diagnostic purpose [6,35]. Motor evoked potentials contralateral to the lesion showed an increase in the excitatory voltage threshold [2,61] and less complex waveforms [2] with lower amplitude [6,61] and prolonged latency [6,61]. Somatosensory evoked potentials were abolished on the side contralateral to the lesion [21]. Nevertheless, transcranial evoked potentials were sometimes useless in case of peripheral nerve lesion [35].
In this review, a good outcome regarding motor function was reported in 23 patients (45%). Among them, the motor deficit fully recovered in 11 patients (21.6%) and recovered partially in 12 patients (23.5%) with a motor strength still ranked at least 3/5 on the mMRC scale. Among the cases of good functional outcome regarding the motor function, they were one case (2%) of dysarthria and one case (2%) of hemianopsia. A bad outcome was reported in 20 patients (39%) : there were 11 cases (22%) of “hemiparesis”, four cases (8%) of “severe hemiparesis” and one case (2%) of “hemiplegia”. There were also two cases (4%) of delayed hemi-parkinsonism where ioflupane (123I) single photon emission computed tomography (DatSCAN™ SPECT) showed a reduced dopamine transporter-specific tracer uptake in the striata contralateral to the initial lesion [15,53]. There were two deaths (4%) in this review. Long-term functional outcome data was missing in eight cases (15.7%).
The long-term functional outcome was not influenced by any of the clinical, radiologic, or surgical criteria that were investigated : an age over 60 years old (p=0.7086), dilated pupils upon admission (p=1); the presence of an ASH (p=0.7555), a CSH (p=0.35) or an EDH (p=1), and a DC preferred over craniotomy (p=0.4888). The only variable that was not significant but showed a trend toward a better motor functional outcome was a GCS of more than 3 upon admission (p=0.1065) (Table 4).
KWNP is a rare clinical and radiologic entity generally encountered after acute and massive side-to-side temporal mass effect. To date, there has been one scoping literature review conducted by Zhang et al. [63] in 2016 concerning 35 case reports and case series for a total of 38 patients suffering from KWNP. This work provided a preliminary description of the clinical presentation, the neuroimaging features, and the long-term functional outcome associated with KWNP. Given the rarity of KWNP, we decided to conduct a systematic literature review in order to allow for a better understanding of these three elements.
Patients suffering from KWNP presented a critical level of midbrain compression, more than half of them being in the coma with pupil anomalies [56]. CT scan of the brain seemed to be the optimal neuroimaging choice in the emergency setting considering its availability and its execution speed. This review was a witness of the paradigm shift in the emergency neurosurgical management of critical intracranial hypertension : the majority of the patients operated on before 2010 benefited from craniotomy, whereas almost half of the cases encountered since then benefited from DC, especially in case of ASH [24]. In this situation, the two objectives of emergency surgical management is to obtain immediate relief of brainstem displacement and to provide long-term control of intracranial hypertension. Hence, the procedure’s choice remains at the discretion of the neurosurgeon taking into account the usual standards concerning a given disease, but also the particularly important mass effect on the brainstem in this unusual syndrome.
At first, a few clarifications concerning the surgical anatomy of the tentorial notch shall be provided in order to fully understand KWNP. The tentorial notch (otherwise called tentorial incisura) is defined as the space delimited between the dorsum sellae anteriorly, the free edges of the cerebellar tentorium laterally and the apex of the notch (reunion of internal cerebral veins and basal veins) posteriorly. It can be divided in three sections antero-posteriorly. The most anterior one contains the basilar artery with its last divisions the SCA and the PCA, and the CN III (or oculomotor nerve). The middle one contains the midbrain; the PCA and the SCA run alongside it, one on each side of the cerebellar tentorium. The posterior one contains the apex of the anterior lobe of the cerebellum.
CN III contains two different fiber tracts. The “extrinsic fibers” arise from the oculomotor nucleus located anterior to periaqueductal grey and constitute the oculomotor tract. The “intrinsic fibers” come from Edinger-Westphal nucleus, located medially from the oculomotor nucleus, and constitute the parasympathetic tract which controls the iris sphincter muscle responsible for producing myosis. These two fiber contingents exit together the midbrain between the cerebral peduncles and pass between the SCA and the PCA which are the last two divisions of the basilar artery, then they run anteriorly and laterally in the interpeduncular cistern to join the roof of the cavernous sinus. The SCA runs laterally along the cerebral peduncle, then it turns posteriorly in the pontopeduncular angle directly below the cerebellar tentorium. The PCA loops backward and upward around the cerebral peduncle along the edge of the tentorial notch, to access the supratentorial space and provide blood supply to the posterior and inferior part of the temporal lobe, the medial part of the occipital lobe, and the thalamus [42,57].
The CST travels in the posterior arm of the internal capsule, then it runs through the middle of the cerebral peduncle (also called crus cerebri) just in front of the substantia nigra (otherwise called locus niger). In the cerebral peduncle, the CST is bordered medially by the frontopontine tract and laterally by parieto-temporo-occipitopontine tract [48] (Fig. 3).
Herniation of the uncus of the parahippocampal gyrus beyond the edge of the cerebellar notch compresses the ipsilateral peduncle and thus stretches the ipsilateral CN III. Hence, uncal herniation is usually responsible for ipsilateral mydriasis caused by the inhibition of the intrinsic part of CN III, and contralateral hemiplegia [58]. In case of massive side-to-side mass effect, the displacement of the brainstem causes the contralateral cerebral peduncle to be compressed and thus notched against the free edge of the tentorium. Hence, authors classically described the KWNP as motor deficit ipsilateral to the lesion associated with radiologic evidence of compression of the contralateral cerebral peduncle against the tentorial notch. Ipsilateral, contralateral and even bilateral mydriasis could occur during KWNP given the importance of lateral displacement of the brainstem and the subsequent stretching of both CN III [47]. In the light of this review, KWNP long-term functional outcome appears more as a clinical spectrum corresponding to the damage to the cerebral peduncle but also to the nervous and vascular surrounding structures [51]. Patients suffering from KWNP can notably present ipsilateral hemiparkinsonism caused by damage to the contralateral substantia nigra [15,53], contralateral cortical blindness related to external compression ischemic stroke of ipsilateral PCA [41], and probably contralateral cerebellar syndrome caused by external compression ischemic stroke of contralateral SCA [34] (Fig. 3).
Some authors have identified that the tentorial notch’s width between the free edges of the tentorium and its length between the dorsum sellae and the apex of the notch presented anatomical variations between individuals [1,12]. In “short” and “narrow” tentorial notches, the lateral part of the cerebral peduncles was already in contact with the free edges of the tentorium in a physiological situation, whereas the cerebral peduncles were surrounded by cerebrospinal fluid in case of “large” tentorial notches. Given these considerations, it is possible that the notching of the contralateral cerebral peduncle against the free edge of the tentorium occurs sooner in case of “narrow” tentorial notch. This anatomical configuration could be an individual predisposing anatomical factor for KWNP.
In a subacute setting, the focal area of cerebral peduncle notched against the free edge of the tentorium displayed the typical features of ischemic stroke with restricted diffusion [9,54] and edema in FLAIR sequence [2,4,14,19-21,34,37,60]. Nevertheless, most of the time the lesion was facing the free edge of the cerebellar tentorium and did not correspond to the systematized anterolateral territory supplied by the PCA, which usually causes hemiparesis or hemiataxia [32]. It did not correspond to the classical neuroimaging features of a midbrain cerebral contusion such as Duret hemorrhage either [25], which harbors spontaneous hyperdensity on CT scan, and hyperintensity in T1-weighted imaging and hypointensity in gradient echo sequence on brain MRI. Indeed, only one patient in this review presented hypointensity on gradient echo imaging [5]. Thus, the most plausible pathophysiological hypothesis for KWNP was a temporary insufficient regional blood supply secondary to mechanical compression against the tentorial notch.
Nevertheless, the association of the midbrain typical features of KWNP and external compression ischemic stroke of the posterior cerebral circulation [34,41] and even the superior cerebellar circulation [34] in a few cases demonstrates that the KWNP is the result of the sudden compression of the complex entanglement of nervous and vascular elements passing through or by the cerebellar notch.
The medium-term and long-term aspect of the midbrain lesion associated with KWNP displayed the neuroimaging features of “white matter hyperintensity (WMH)” [18] which corresponds to hyperintensity in T2 [8,15,27,44] and FLAIR sequence [44] after ischemic stroke. Hypointensity in T1-weighted imaging indicated cerebral atrophy [27]. Interestingly, the WMH were strictly located in the cerebral peduncle and limited posteriorly by the substantia nigra, whereas patients presented more extended FLAIR hyperintensity in the acute setting [2,19,21,37,41,60]. Thus, it seems that FLAIR hyperintensity in the acute setting corresponds to compression ischemia centered on the cerebral peduncle and compression cerebral edema extending to the surrounding structures (Fig. 2).
Because more than half of the patients in this review suffered from ASH, we naturally assumed that the usual prognostic criteria for ASH such as the patient’s age and a low GCS upon admission could also influence the motor functional prognosis in KWNP. On the same way, we tried to identify whether the aggressiveness of the disease and the emergency operative technique that was employed were associated with the motor functional outcome. With the benefit of hindsight, we chose criteria which are relevant for the criteria that were chosen in this work to we used criteria which were relevant for the GOS to try to predict the motor functional outcome. The fact is that in this review, 49 patients (96%) presented a good outcome based on the GOS. Thus, we think that future studies will have to focus on more personalized criteria that could be associated with good motor function recovery. One point worth exploring would be to assess whether there is a tentorial notch width difference in KWNP patients compared with controls [1,12].
This study presents a few limits inherent to its retrospective nature. First, this was a retrospective study focused on case reports. Consequently, there was a reporting bias in favor of patients with a good outcome and a measurement bias during the collection of the information. What is more, the motor strength was reported heterogeneously among the case reports which leads inevitably to a measurement bias.
On the other hand, this was the largest study to date concerning KWNP. This work has shed light on the presumed pathophysiology of the syndrome and has provided a thorough description of the neuroimaging patterns encountered. No significant prognostic factors for long-term motor function have been identified with epidemiological, clinical and surgical criteria, which opens the door to the study of more personalized elements such as the individual width of tentorial notch as a potential predisposing factor.
KWNP is a well-known yet incompletely understood clinical and radiologic syndrome which occurs when uncal herniation secondary to an intracerebral lesion induces important brainstem displacement with compression of the contralateral cerebral peduncle against the free edge of cerebellar tentorium. To date, KWNP has been essentially described as the association of motor deficit ipsilateral to the lesion and radiologic or neurophysiological test evidence of damage to the contralateral cerebral peduncle. This review sheds light on the fact that KWNP is a regional compression syndrome with lesion to the cerebral peduncle, but also to surrounding structures such as the thalamus, the internal capsule and the splenium of the corpus callosum, and even to the arteries passing nearby. MRI appears to be the gold standard for the long-term assessment of white matter hyperintensities located in the cerebral peduncle. The motor functional outcome is good in half of the cases; nevertheless, no predisposing factor has been identified in this review. The GCS upon admission seems to be the most promising factor and calls for more powerful studies. Last, the width of the tentorial notch could be an anatomical predisposing factor worth exploring in future anatomical MRI-bases studies. This being said, multicentric prospective trials will be very difficult to conduct on such a rare entity.
Notes
Author contributions
Conceptualization : NB; Data curation : NB, PJC, GB, SC, AD, PHR; Formal analysis : NB, PJC, GB, SC, AD, PHR; Methodology : NB; Project administration : NB, AD, PHR; Visualization : NB, PJC, GB, SC, AD, PHR; Writing - original draft : NB, PJC, GB, SC, AD, PHR; Writing - review & editing : NB, AD, PHR
Supplementary materials
The online-only data supplement is available with this article at https://doi.org/10.3340/jkns.2022.0002.
Supplementary Table 1
Systematic review focused on Kernohan-Woltman phenomenon in MEDLINE database : patients’ complete data
References
1. Adler DE, Milhorat TH. The tentorial notch: anatomical variation, morphometric analysis, and classification in 100 human autopsy cases. J Neurosurg. 96:1103–1112. 2002.
2. Binder DK, Lyon R, Manley GT. Transcranial motor evoked potential recording in a case of Kernohan’s notch syndrome: case report. Neurosurgery. 54:999–1002. 2004.
3. Çabalar M, Başkan F, Erdoğan HA, Bulut A, Yayla V. Kernohan-Woltman notch phenomenon secondary to a subdural hematoma in a young man. Clin Neuroradiol. 25:435–436. 2015.
4. Carlstrom LP, Perry A, Puffer RC, Graffeo CS, Reuter PJ, Fogelson JL, et al. A puzzling exam: Kernohan’s notch reimaged. J Clin Neurosci. 80:336–337. 2020.
5. Carrasco R, Pascual JM, Navas M, Martínez-Flórez P, Manzanares-Soler R, Sola RG. Kernohan-Woltman notch phenomenon caused by an acute subdural hematoma. J Clin Neurosci. 16:1628–1631. 2009.
6. Carrasco-Moro R, Abreu-Calderón F, de Blas-Beorlegui G, Pascual JM, Ley-Urzaiz L. Kernohan-Woltman notch phenomenon. Rev Clin Esp (Barc). 214:e97–e99. 2014.
7. Chang GY. Contusional Kernohan notch phenomenon. Neurol Clin Pract. 6:553–554. 2016.
8. Clement VL, Sherer M. Neuropsychological findings in a patient with Kernohan’s notch. Appl Neuropsychol. 3:55–57. 1996.
9. Codd PJ, Agarwalla PK, Berry-Candelario J, Nahed BV. Kernohan-Woltman notch phenomenon in acute subdural hematoma. JAMA Neurol. 70:1194–1195. 2013.
10. Cohen AR, Wilson J. Magnetic resonance imaging of Kernohan’s notch. Neurosurgery. 27:205–207. 1990.
11. Compston A. Aids to the investigation of peripheral nerve injuries. Brain. 133:2838–2844. 2010.
12. Corsellis JA. Individual variation in the size of the tentorial opening. J Neurol Neurosurg Psychiatry. 21:279–283. 1958.
13. Dibble CF, Wemhoff MP, Ibrahim T, Sasaki-Adams D, Solander S, Germanwala AV. Ruptured arteriovenous malformation presenting with Kernohan’s notch. Case Rep Neurol Med. 2015:921930. 2015.
14. Eesa M, Bell K. Kernohan-Woltman notch phenomenon. J Trauma. 69:1634. 2010.
15. Evans AH, Gacinovic S, Costa DC, Lees AJ. Parkinsonism due to Kernohan notch: clinical, structural, and functional imaging correlates. Neurology. 62:2333–2334. 2004.
16. Evans LR, Jones J, Lee HQ, Gantner D, Jaison A, Matthew J, et al. Prognosis of acute subdural hematoma in the elderly: a systematic review. J Neurotrauma. 36:517–522. 2019.
17. Fujimoto A, Sato H, Katayama W, Nakai K, Tsunoda T, Kobayashi E, et al. Kernohan’s phenomenon associated with left ruptured occipital arteriovenous malformation. J Clin Neurosci. 11:444–446. 2004.
18. Georgakis MK, Duering M, Wardlaw JM, Dichgans M. WMH and long-term outcomes in ischemic stroke: a systematic review and meta-analysis. Neurology. 92:e1298–e1308. 2019.
19. Gimarc K, Massagli TL. Kernohan-Woltman notch phenomenon in two patients with subdural hematoma and ipsilateral hemiparesis. Am J Phys Med Rehabil. 99:1195–1196. 2020.
20. Giménez-Pando J, Cabezudo-Artero JM, Fernández-Portales I, Rodríguez-Sánchez JA, Gómez-Perals L, Lorenzana L, et al. Contralateral mesencephalic injury due to tentorial uncal herniation. magnetic resonance imaging of the Kernohan’s notch. Neurocirugia (Astur). 15:384–387. 2004.
21. Gobert F, Baars JH, Ritzenthaler T, Afathi M, Boulogne S, André-Obadia N, et al. Diagnosing Kernohan-Woltman notch phenomenon by somatosensory evoked potentials in intensive care unit. Clin Neurophysiol. 129:254–257. 2018.
22. Groeneveld A, Schaltenbrand G. Ein fall von duraendotheliom über der großhirnhemisphäre mit einer bemerkenswerten komplikation: läsion des gekrenzten pes pedunculi durch druck auf den rand des tentoriums. Deutsche Zeitschrift f Nervenheilkunde. 97:32–50. 1927.
23. Hamada H, Kuwayama N, Endo S, Takaku A. Detection of Kernohan’s notch on magnetic resonance imaging in a patient with acute subdural haematoma. Br J Neurosurg. 14:498–499. 2000.
24. Hutchinson PJ, Kolias AG, Timofeev IS, Corteen EA, Czosnyka M, Timothy J, et al. Trial of decompressive craniectomy for traumatic intracranial hypertension. N Engl J Med. 375:1119–1130. 2016.
25. Ishizaka S, Shimizu T, Ryu N. Dramatic recovery after severe descending transtentorial herniation-induced duret haemorrhage: a case report and review of literature. Brain Inj. 28:374–377. 2014.
26. Itoyama Y, Fujioka S, Ushio Y. Kernohan’s notch in chronic subdural hematoma: findings on magnetic resonance imaging. J Neurosurg. 82:645–646. 1995.
27. Iwama T, Kuroda T, Sugimoto S, Miwa Y, Ohkuma A. MRI demonstration of Kernohan’s notch: case report. Neuroradiology. 34:225–226. 1992.
28. Jang SG, Pyun SB. Diffusion tensor tractography in two cases of Kernohan-Woltman notch phenomenon. Ann Rehabil Med. 37:879–885. 2013.
29. Jang SH, Chang CH, Jung YJ, Seo JP. Locked-in syndrome due to transtentorial herniation and Kernohan notch phenomenon. Am J Phys Med Rehabil. 96:e77. 2017.
30. Jones KM, Seeger JF, Yoshino MT. Ipsilateral motor deficit resulting from a subdural hematoma and a Kernohan notch. AJNR Am J Neuroradiol. 12:1238–1239. 1991.
31. Kernohan JW, Woltman HW. Incisura of the crus due to contralateral brain tumor. Arch NeurPsych. 21:274–287. 1929.
32. Kim JS, Kim J. Pure midbrain infarction: clinical, radiologic, and pathophysiologic findings. Neurology. 64:1227–1232. 2005.
33. Kole MK, Hysell SE. MRI correlate of Kernohan’s notch. Neurology. 55:1751. 2000.
34. Leever JD. Unilateral cerebral herniation resulting in combined contralateral superior cerebellar artery territory infarction and mesencephalic injury: two cases of a severe unrecognized variant of Kernohan notch phenomenon? Radiol Case Rep. 15:250–253. 2020.
35. Lim N, Lee G, Won KH, Kang JS, Lee S, Cho Y, et al. Usefulness of diffusion tensor imaging in unexplained ipsilateral hemiplegia. Korean J Neurotrauma. 17:61–66. 2021.
36. Mastronardi L, Puzzilli F, Ruggeri A, Guiducci A. Magnetic resonance imaging findings of Kernohan-Woltman notch in acute subdural hematoma. Clin Neurol Neurosurg. 101:122–124. 1999.
37. Mejía Kattah J, Vilá Barriuso E, García Bernedo C, Gallart Gallego L. Kernohan-Woltman notch phenomenon secondary to a cranial epidural hematoma. Rev Esp Anestesiol Reanim. 61:332–335. 2014.
38. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6:e1000097. 2009.
39. Mohseni M, Habibi Z, Nejat F. Contralateral superior cerebellar artery syndrome: a consequence of brain herniation. J Korean Neurosurg Soc. 60:362–366. 2017.
40. Moon KS, Lee JK, Joo SP, Kim TS, Jung S, Kim JH, et al. Kernohan’s notch phenomenon in chronic subdural hematoma: MRI findings. J Clin Neurosci. 14:989–992. 2007.
41. Namura S, Kang Y, Matsuda I, Kamijyo Y. Magnetic resonance imaging of sequelae of temporal lobe herniation secondary to traumatic acute subdural hematoma: Kernohan’s notch and posterior cerebral artery territory infarctions contralateral to the supratentorial lesion--case report. Neurol Med Chir (Tokyo). 37:32–35. 1997.
42. Ono M, Ono M, Rhoton AL Jr, Barry M. Microsurgical anatomy of the region of the tentorial incisura. J Neurosurg. 60:365–399. 1984.
43. Openshaw H. Kernohan notch lesion after spinal tap. Neurology. 64:1631. 2005.
44. Oster JM, Jones HR Jr, Hildenbrand P, Tronic B, Cosgrove GR. Reversible Kernohan notch. Neurology. 68:368. 2007.
45. Ozturk S, Ozturk Y, Ocal O. The first case of Kernohan-Woltman notch phenomenon caused by epidural hematoma in a pediatric patient. Pediatr Neurosurg. 52:181–184. 2017.
46. Panikkath R, Panikkath D, Lim SY, Nugent K. Kernohan’s notch: a forgotten cause of hemiplegia-CT scans are useful in this diagnosis. Case Rep Med. 2013:296874. 2013.
47. Ritter AM, Muizelaar JP, Barnes T, Choi S, Fatouros P, Ward J, et al. Brain stem blood flow, pupillary response, and outcome in patients with severe head injuries. Neurosurgery. 44:941–948. 1999.
48. Samii M, Carvalho GA, Tatagiba M, Matthies C, Vorkapic P. Meningiomas of the tentorial notch: surgical anatomy and management. J Neurosurg. 84:375–381. 1996.
49. Schedler P, Geary S. Kernohan’s notch phenomenon: a case study. J Neurosci Nurs. 34:158–159. 2002.
50. Simonin A, Levivier M, Nistor S, Diserens K. Kernohan’s notch and misdiagnosis of disorders of consciousness. BMJ Case Rep. 2014:bcr2013202094. 2014.
51. Sunderland S. The tentorial notch and complications produced by herniations of the brain through that aperture. Br J Surg. 45:422–438. 1958.
52. Ten Harmsen BL, De Kleermaeker FGCM, De Leeuw C, Van Dijk G. Neuro-images Kernohan-Woltman notch phenomenon in acute subdural hematoma. Acta Neurol Belg. 120:189–190. 2020.
53. Ueda M, Tsunogae M, Saito H, Suzuki T, Ota T. Delayed hemiparkinsonism associated with Kernohan’s notch in a patient with a ruptured arteriovenous malformation. Intern Med. 60:309–313. 2021.
54. Uesugi S, Suehiro E, Nakayama H, Suzuki M. Diffusion-weighted magnetic resonance imaging in a case of Kernohan’s notch phenomenon. Acta Neurochir (Wien). 152:1809–1810. 2010.
55. Velasquez JL, Sanz ER, Lasaosa SS, Del Val JL. Kernohan’s notch. Pract Neurol. 15:221. 2015.
56. Wijdicks EF. Uncal herniation in acute subdural hematoma: point of no return. Arch Neurol. 59:305. 2002.
57. Wijdicks EF. Neurological picture. Acute brainstem displacement without uncal herniation and posterior cerebral artery injury. J Neurol Neurosurg Psychiatry. 79:744. 2008.
58. Wijdicks EFM. Through the eyes of monkeys: questions about uncal herniation. Neurocrit Care. 35:573–576. 2021.
59. Wolf RF, Ter Weeme CA, Krikke AP. Kernohan’s notch and misdiagnosis. Lancet. 345:259–260. 1995.
60. Yogarajah M, Agu CC, Sivasambu B, Mittler MA. HbSC disease and spontaneous epidural hematoma with Kernohan’s notch phenomena. Case Rep Hematol. 2015:470873. 2015.
61. Yoo WK, Kim DS, Kwon YH, Jang SH. Kernohan’s notch phenomenon demonstrated by diffusion tensor imaging and transcranial magnetic stimulation. J Neurol Neurosurg Psychiatry. 79:1295–1297. 2008.
62. Zafonte RD, Lee CY. Kernohan-Woltman notch phenomenon: an unusual cause of ipsilateral motor deficit. Arch Phys Med Rehabil. 78:543–545. 1997.
63. Zhang CH, DeSouza RM, Kho JS, Vundavalli S, Critchley G. KernohanWoltman notch phenomenon: a review article. Br J Neurosurg. 31:159–166. 2017.
Fig. 1.
Medline based systematic review on Kernohan-Woltman notch phenomenon. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart. Adopted from Moher et al. [38].
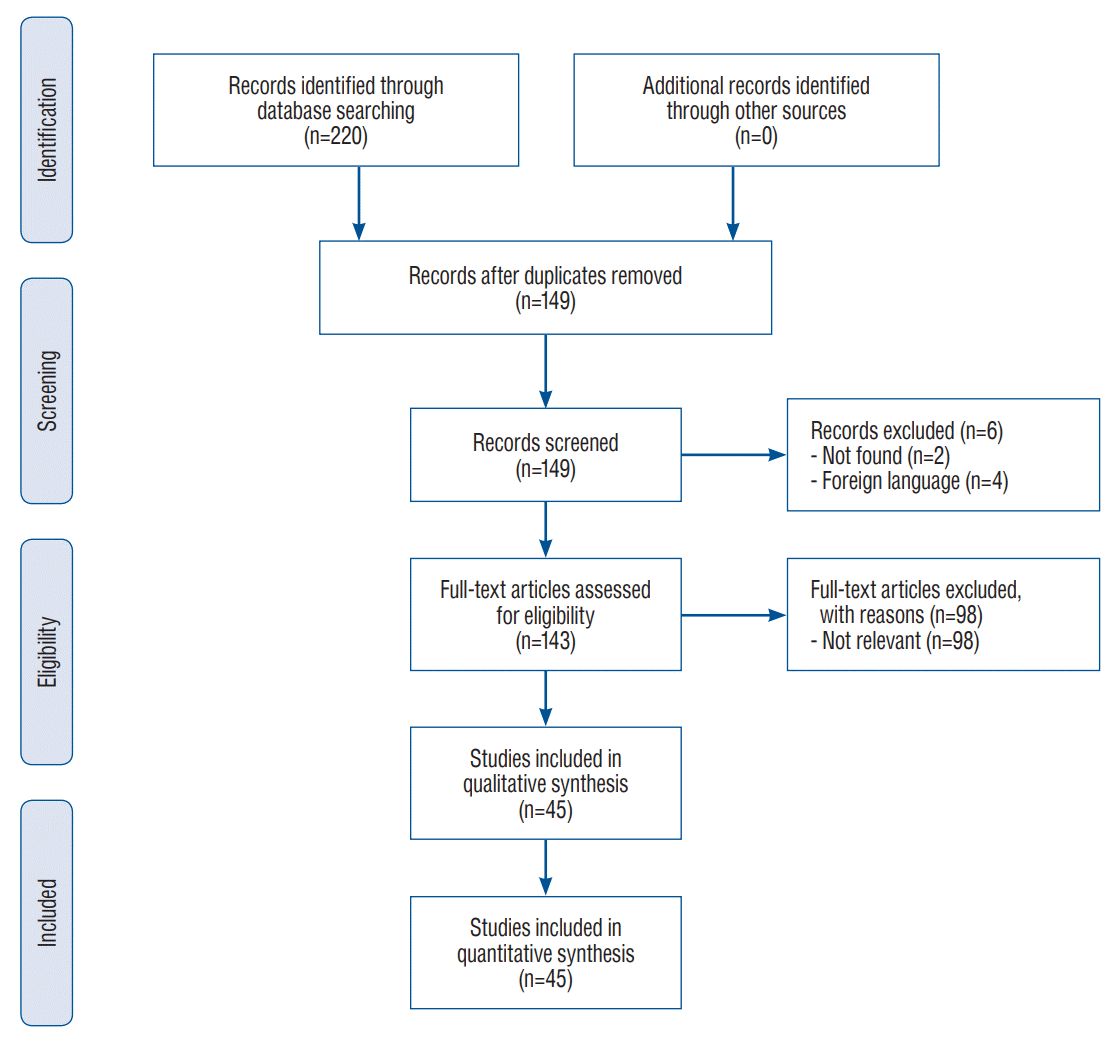
Fig. 2.
Typical features of Kernohan-Woltman notch phenomenon on brain magnetic resonance imaging (MRI). A and B : T2-weighted MRI shows hyperintensity in the left cerebral peduncle facing the tentorial notch (courtesy of Simonin et al. [50]). C : MRI in fluid-attenuated inversion recovery sequence displays hyperintensity in the left cerebral peduncle (white arrow) (courtesy of Yogarajah et al. [60]). D : MRI diffusion-weighted imaging displays hyperintensity in the right cerebral peduncle (white arrow) (courtesy of Chang [7]). E : T2-weighted MRI 2 years after injury displays white matter hyperintensity in the substantia nigra of the left cerebral peduncle (black arrowhead) (courtesy of Ueda et al.[53]). F : MRI in diffusion tensor imaging with tractography displays interruption of the right corticospinal tract 8 weeks after injury (courtesy of Jang and Pyun [28]). G : Ioflupane (123I) single photon emission computed tomography (DatSCAN™ SPECT) 2 years after trauma shows a reduced dopamine transporter-specific tracer uptake in the left striata (for credits see E).
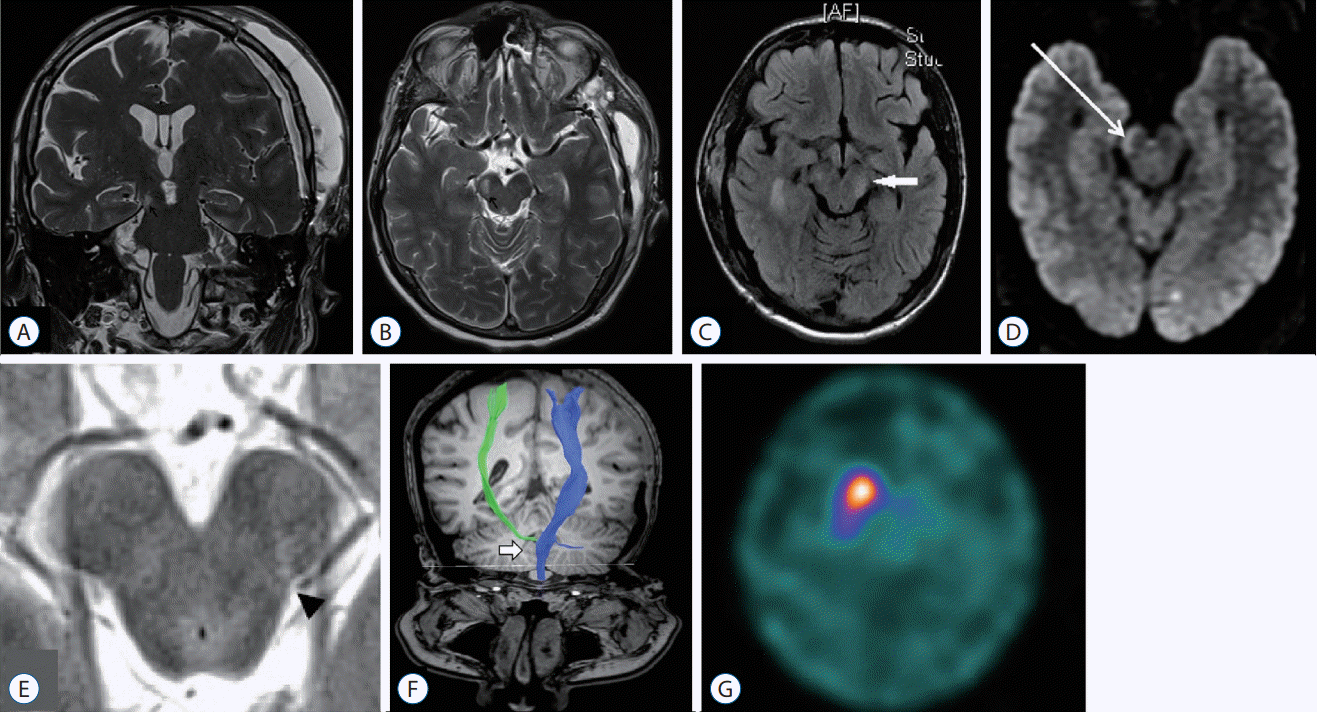
Fig. 3.
Artistic view of Kernohan-Woltman notch phenomenon caused by a right-sided acute subdural hematoma in coronal (left illustration) and axial view (right illustration). Please note the interruption of the contralateral corticospinal tract (green) because of direct compression against the free edge of the cerebellar tentorium. The posterior cerebral artery is compressed between the herniated temporal uncus (red arrowhead) and the midbrain, and the superior cerebellar artery between the cerebral peduncle and the inferior face of the tentorium (purple arrowhead). In axial view, ipsilateral CN III is stretched by the brainstem displacement. Nathan BEUCLER is the corresponding author of this article and certifies that he is the artist who performed the drawings illustrated in Fig. 3. The authorization to publish this Figure is provided as Supplementary Table 1. PCA : posterior cerebral artery, SCA : superior cerebellar artery, CN II : second cranial nerve, optic nerve, ACP : anterior clinoid process, CN III : third cranial nerve, oculomotor nerve.
Table 1.
Mechanism of injury and etiology
Table 2.
Neurological status upon admission
Table 3.
Aspect of Kernohan-Woltman associated lesion in the contralateral cerebral peduncle on brain MRI
Table 4.
Predisposing factors of Kernohan-Woltman notch phenomenon




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download