Abstract
Clinical studies on neuromodulation intervention for trigeminal neuralgia have not yet shown promising results. This might be due to the fact that the pathophysiology of chronic trigeminal neuropathy is not yet fully understood. Chronic trigeminal neuropathy includes trigeminal autonomic neuropathy, painful trigeminal neuropathy, and persistent idiopathic facial pain. This disorder is caused by complex abnormalities in the pain processing system, which is comprised of the affective, emotional, and sensory components, rather than mere abnormal sensation. Therefore, integrative understanding of the pain system is necessary for appropriate neuromodulation of chronic trigeminal neuropathy. The possible neuromodulation targets that participate in complex pain processing are as follows : the ventral posterior medial nucleus, periaqueductal gray, motor cortex, nucleus accumbens, subthalamic nucleus, globus pallidus internus, anterior cingulate cortex, hypothalamus, sphenopalatine ganglion, and occipital nerve. In conclusion, neuromodulation interventions for trigeminal neuralgia is yet to be elucidated; future advancements in this area are required
Medically intractable trigeminal neuralgia is treatable with conventional surgical modalities, such as microvascular decompression (MVD), percutaneous radiofrequency rhizotomy, balloon compression, and stereotactic radiosurgery. While the majority of patients benefits from these modalities, a significant proportion of patients are refractory to the primary surgical modalities. Such groups can be categorized as chronic trigeminal neuropathy, which include atypical facial pain and anesthesia dolorosa.
Several neuromodulation techniques such as motor cortex stimulation (MCS) and deep brain stimulation (DBS), have been attempted for the treatment of such conditions [7,21,30,43,51]. Early clinical results had shown promising results. However, later clinical studies found that those early clinical benefits were not sustained in long-term observations [43].
However, all such therapeutic strategies failed in a significant volume of patients. Therefore, instead of attempting to answer whether neuromodulation is effective for chronic trigeminal neuropathy, the present review aimed to gain insight into whether neuromodulation techniques hold promise in the treatment of intractable chronic trigeminal neuropathy.
In the cases of failure of conventional therapeutic modalities, including medical and surgical treatments, in patients initially diagnosed with trigeminal neuralgia, physicians should consider the possible explanations such as initial misdiagnosis, progression of trigeminal neuralgia, or trigeminal neuropathy arising from an adverse event of conventional treatments.
According to the International Classification of Headache Disorders (ICHD)-3 beta, trigeminal neuralgia is diagnosed with typical symptoms such as presence of severe paroxysmal pain with a refractory period between pain attacks occurring unilaterally in accordance with trigeminal nerve distribution areas [81].
There are sub-classifications of trigeminal neuralgia. Classical trigeminal neuralgia can be defined as when an evident offending vessel causing typical facial pain at the trigeminal nerve root exit zone [8]. If typical facial pain originates from other factors such as tumor and vascular malformations conflicting the trigeminal nerve root, this condition is defined as secondary trigeminal neuralgia. Both classical and secondary trigeminal neuralgia are expected to be good indicative for surgical treatment such as MVD or removal of intracranial lesions [8]. However, the presence of typical facial pain of trigeminal neuralgia does not guarantee the existence of an offender, and this condition is termed idiopathic trigeminal neuralgia. In addition, if there is background pain in combination with the typical symptoms of trigeminal neuralgia, it is separately classified into a concomitant continuous pain group.
Thus, the most important feature for the diagnosis of trigeminal neuralgia is the clinical observation of typical facial pain [20]. However, clinical situations in the real world are more complex than those expected, as practically, typical pain is not always easily distinguished from atypical facial pain, and patients with facial pain often describe their pain in ambiguous ways. Furthermore, pain in the trigeminal dermatomes may be observed in other pain syndromes that have different pathogenesis and clinical courses. In other words, the diagnosis of trigeminal neuralgia should be based on observation of the typical symptoms of patient’s pain; the presence of the symptoms cannot be exclusively correlated to the existence of trigeminal neuralgia. Therefore, clinicians should always consider the possibility that other facial pain syndromes could be misdiagnosed as trigeminal neuralgia especially in an early stage of the disease.
There are three categories of craniofacial pain syndrome possibly confused with typical pain of trigeminal neuralgia in ICHD-3 beta : trigeminal autonomic cephalalgia (TAC), painful trigeminal neuropathy, and persistent idiopathic facial pain [20].
The most well-known subcategory of TAC is cluster headache, which is characterized by intermittent unilateral periorbital pain accompanied by autonomic symptoms, such as nasal congestion and conjunctival injection [69,75]. In cluster headache, as the periorbital area overlaps with the nerve distribution of the ophthalmic nerve (V1), it may be misdiagnosed as trigeminal neuralgia at the V1 area. Other facial pain syndromes included in TAC are paroxysmal hemicranias, short-lasting unilateral neuralgiform headache attack, and hemicranias continua. Although there are small differences in the frequencies or duration of pain, all of these syndromes have common features in that the main symptom is severe paroxysmal periorbital pain in combination with the autonomic nervous symptoms [35].
Painful trigeminal neuropathy indicates neuropathic pain along the trigeminal nerve distribution caused by herpes zoster, trauma, or other etiologies. Symptoms of painful trigeminal neuropathy are common symptoms of neuropathic pain such as hyperalgesia, allodynia, dysesthesia and paresthesia, which are accompanied by dysfunction of the trigeminal nerve [20,73].
Persistent idiopathic facial pain refers to pain previously called atypical facial pain. While trigeminal neuralgia is characterized by intermittent paroxysmal facial pain with refractory period between attacks, persistent pain without a definite refractory period is the hallmark of the persistent idiopathic facial pain [45]. As described above, however, persistent pain between paroxysmal attacks might be presented in patients with trigeminal neuralgia in the concomitant continuous pain group [45,73]. The persistent idiopathic facial pain could also have waxing and waning patterns. Practically, difficult cases in which trigeminal neuralgia and persistent idiopathic facial pain cannot be clearly distinguished are presented.
Hence, trigeminal neuralgia may be initially incorrectly diagnosed. Even though a physician is aware that a patient's symptoms may be vague and mixed with typical and atypical facial pain symptoms, accurate diagnosis is not always possible. Therefore, in actual clinical practice, a clinician often judges the diagnosis of patients with facial pain in terms of the progress in response to a particular treatment. Therefore, an appraisal that there was an initial misdiagnosis of trigeminal neuralgia may just be an explanation for the unsuccessful treatment outcomes rather than contemplation based on principles of pathophysiology.
As described above, the ICHD-3 beta is referred for diagnoses based on symptomatology rather than the pathophysiology of craniofacial pain syndromes, including trigeminal neuralgia. However, similar to other facial pain symptoms, it is also true that the symptoms of the patients with trigeminal neuralgia may change over time.
The first treatment of choice for trigeminal neuralgia is carbamazepine, and most patients are reported to respond well to this medicine [20]. However, this primary medicine does not work in many patients when trigeminal neuralgia is recurred [8,45]. In case of medically intractable classical trigeminal neuralgia, MVD should be considered a surgical treatment option. It has been reported that immediate pain relief rate of the MVD ranged between 80% and 98% [57]. However, there are significant recurrent rate after the MVD in a long-term follow-up ranging 9–30%. Many reports addressed that reoperation for recurred trigeminal neuralgia had failed to find a newly developed vascular conflict.
Similarly, the percutaneous radiofrequency rhizotomy of the gasserian ganglion has been known to be effective for medically intractable trigeminal neuralgia with a success rate between 80% and 100% [57]. However, recurrence rate of the radiofrequency rhizotomy was as high as 75% in a long-term observation. It is considered that an ablated ganglion does not regenerate; therefore, it is reasonable that the change of pathophysiologic condition related to facial pain is responsible for the recurrence of facial pain.
Anesthesia dolorosa, which is caused by iatrogenic damage of trigeminal nerve, is another type of disorder as a result of surgical treatment of trigeminal neuralgia. Generally, damage of the neuronal component could cause abnormal signal transmission above the level of injury. When this abnormal signal transmission becomes permanent, neuropathic pain may develop along with abnormal neural activities. In ICHD-3 beta, anesthesia dolorosa is classified as a type of painful trigeminal neuropathy. It is accompanied by the symptoms of typical neuropathic pain, such as hyperalgesia, allodynia, hypoesthesia, spontaneous pain and paresthesia. In many cases, a patient with anesthesia dolorosa usually complaints of severe pain on the face that is typically resistant to all aspect of conventional therapeutic strategies.
Traditional hypothesis explaining pathogenesis of trigeminal neuralgia is as follows : demyelination is caused by vascular or other causative factors, conflicting the nerve root exit zone of the trigeminal nerve. The demyelination is considered to evoke ephaptic transmission and ectopic impulse generation, causing typical facial pain to occur. This concept addresses events between the gasserian ganglion and the trigeminal nucleus, while events proximal and distal to this region are kept out of interest. It can be assumed that chronic trigeminal neuropathy caused by pathogenesis other than the traditional hypothesis may not be improved by conventional treatment. For more discussion on neuromodulations of the chronic trigeminal neuropathy failed by conventional treatments, the neural networks and its role above the trigeminal nuclei should be considered.
Centrally mediated abnormal nociception is yet to be elucidated. Role of brain activity in an intractable facial pain is still under active investigations. New knowledge on how chronic intractable pain is processed and manifested opens hitherto unknown way for the developments of neuromodulation for facial pain. In this section, nociceptive network of the central nervous system is reviewed in an integrative order.
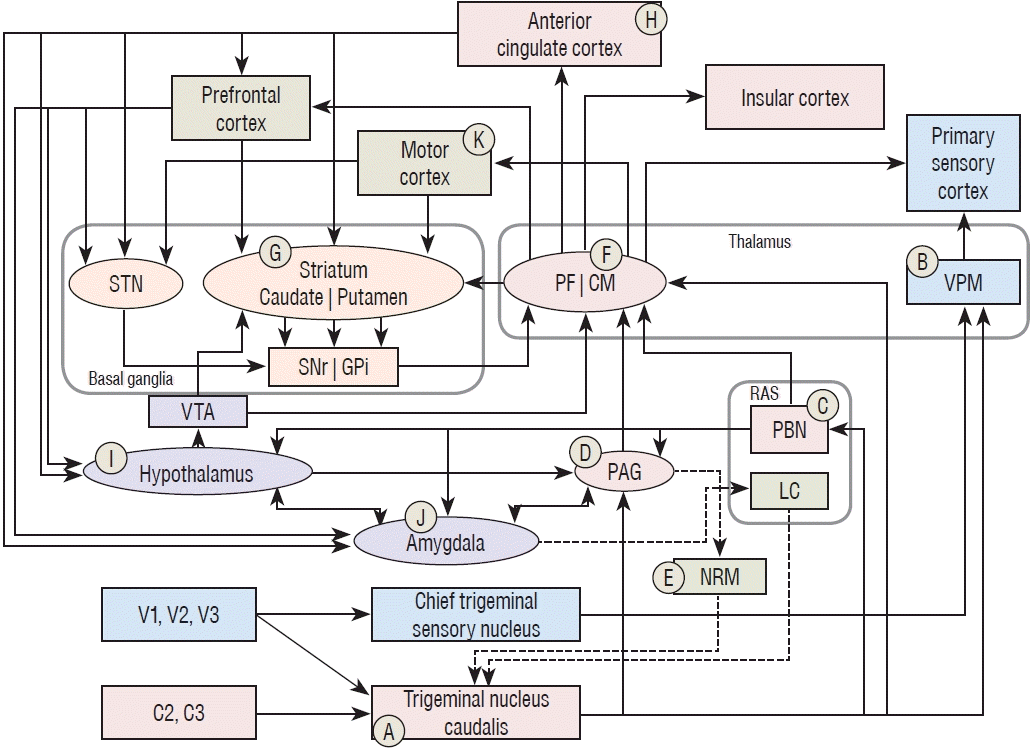
Craniofacial sensory information is conducted via the trigeminal and occipital nerves. Facial sensory information is delivered via three trigeminal nerve branches (V1, V2, and V3), of which the primary sensory neuron is located at the gasserian ganglion. Sensory information of meninges travel through meningeal afferents, which is joined together with the V1 branch and activates the meningeal afferents, causing referred pain on the V1 area. Primary sensory neurons innervating the occipital and parietal area are located in the C2 and C3 ganglia, which branch the greater and lesser occipital nerves. Like other somatosensory systems, craniofacial sensation is also divided into informational sense (also known as discriminative sense) and noxious sense (also known as pain and temperature sense), which travel through the distinct pathway beyond the primary sensory neurons.
The myelinated Aβ fiber from the gasserian ganglion synapses with the chief trigeminal sensory nucleus, where the second-order sensory neuron for the discriminative sensation from the trigeminal nerve is located. The projection fiber from the chief trigeminal sensory nucleus is decussated, and ascends via the contralateral trigeminal lemniscus to the ventral posterior medial (VPM) nucleus of the thalamus, which is the third-order sensory neuron. Then VPM relays sensory information of the face to the primary somatosensory cortex on the parietal lobe, which completes recognition of discriminative sensation.
In contrast, a potentially noxious sense such as pain and temperature travels via the Aδ and C fibers, and it synapses with its second-order sensory neuron, the trigeminal nucleus caudalis. Interestingly, this nucleus is a rostral extension of the dorsal horn of the spinal cord that is located between the cervicomedullary junction and C2-3. Due to this structural feature, nociception from the trigeminal nerve, and C2 and C3 synapse together at the trigeminal nucleus caudalis as their common second-order sensory neuron of the head and face (Ⓐ in Fig. 1).
Pain is composed of three dimensions: sensory, affective, and motivational [68]. From the trigeminal nucleus of caudalis, the nociceptive information travels through different pathways according to the three dimensions. The sensory dimension of pain directs to the VPM, then to the primary somatosensory cortex like the discriminative sensory pathway, which recognizes a presence of potential tissue damage (Ⓑ in Fig. 1).
Recognizing a sensation is not enough; however, for establishing pain, as pain is an unpleasant and emotional sensory experience, affective and motivational dimension must be presented. From the trigeminal nucleus caudalis, nociceptive information ascends and synapses to the three nuclei : to the periaqueductal gray (PAG), parabrachial nucleus (PBN), and intralaminar thalamic nuclei [68,78,82]. Among the several intralaminar thalamic nuclei, the centromedian parafascicular nucleus (CM/PF) is the largest nucleus, and coordinates many important brain functions [33]. Hence the CM/PF will be discussed in this section as a representative nucleus of the intralaminar thalamic nuclei. It can be said that the second-order nociceptive neuron is located at the CM/PF because the PAG and PBN also relay nociceptive signals from the trigeminal nucleus caudalis to the intralaminar thalamic nuclei [24,33,68,82]. Finally nociception reaches to the anterior cingulate cortex (ACC), and insular cortex which are considered the center of affective and motivational dimension of pain [24,58]. The system for perceiving the affective and motivational dimension of pain, i.e., from primary sensory neurons via the PAG, PBN, and CM/PF to the ACC and insular cortex, is called the medial pain system [68,82].
As mentioned above, for any sensation in the face to be recognized as pain, not only recognition of tissue damage in the primary sensory cortex but the recognition of unpleasantness and negative emotion in the ACC and insular cortex must also be presented [58]. In other word, the VPM and somatosensory cortex recognize presence of potential tissue damage, and the CM/PF, ACC and insular cortex impose what that potential tissue damage means to the individual. Therefore, it is very important to understand hedonic process of pain that is operated in brain networks consisting of afferent and efferent connections with the ACC and insular and their output structures for discussing neuromodulation of intractable pain syndromes.
The PBN (Ⓒ in Fig. 1) receives collaterals from the trigeminal nucleus caudalis, nociceptive signals from other parts of the body, and visceral sensory information from internal organs via the nucleus tractus solitaries [5,6,16,61,68]. The PBN integrates those nociceptive and visceral sensory information and relays to the CM/PF, hypothalamus, amygdala, and basal forebrain, by which brain activity is coordinated appropriately against noxious stimulation [9,25,77]. The PBN also send output to the PAG, which participates in the descending pain modulation system discussed below [13,16,55].
The PAG (Ⓓ in Fig. 1) receives nociceptive input not only from the trigeminal nucleus caudalis but also from the PBN, hypothalamus, amygdala, prefrontal cortices (PFC), and ACC where affective and motivational aspects of pain are processed [5,6,68,77,79]. The PAG plays the major site of emotional motor system, which integrates affective and motivational pain information, and returns emotional reactions such as fight or flight, heart rate, blood pressure, and vocalization to each part of the body [68,78]. Furthermore, the PAG relays integrative signal to the nucleus raphe magnus (Ⓔ in Fig. 1) where the descending pain modulation system operates toward the spinal cord dorsal horn [13,16,55,78,83].
The CM/PF (Ⓕ in Fig. 1) plays a key role in the transmission of the affective and emotional component of pain from the spinal cord to the cerebral cortex [33,82]. The noxious signal may be delivered to the CM/PF directly from the spinal cord or indirectly via the PAG and PBN [16,68,82]. The CM/PF relays noxious signal to the ACC, insular cortices and PFC, which participates the perception of affective and emotional aspects of pain [64,68,82]. The CM/PF also sends large amount of output signal to the striatum via the thalamostriatal circuit, which is an important route connecting the basal ganglia [72]. The CM primarily mediates the sensorimotor aspect of pain, while the PF formally participates in the orchestration of the affective and motivational aspect of pain in between the brainstem, cerebral cortex, and basal ganglia [64].
Furthermore, the ACC, PFC, and motor cortex project their collaterals to the striatum (Ⓖ in Fig. 1) with partially interdigitated fashion in functional regions [14,56]. By these corticostriatal projections, cortical activities stimulate the basal ganglia system that encodes procedural memories of sensorimotor, associated, and limbic function of the brain [22]. The CM/PF receives striatal outputs via the globus pallidus internus (GPi) and substantia nigra pars reticulata in a double negative modulation system, i.e., signal of gamma aminobutyric acid [56]. The CM/PF sends orchestrated signals back to the striatum via the thalamostriatal circuit that constitutes the thalamostriatal feedback loop [33,82]. Through the connections with brainstem, the basal ganglia, and cerebral cortex, the CM/PF is engaged in processing of nociception and pain response, and polysensory integration in general, which is considered to be a gate control function [33].
In conjunction with the insular cortex, the ACC (Ⓗ in Fig. 1) recognizes the unpleasantness of pain and integrates emotional and affective components, for the purpose of having a role in planning a physical response to pain [68,79]. Neurons in the ACC respond mainly to noxious stimuli but they do not recognize a location of the injured body. Emotional response is more prominent in more anterior aspects of the ACC, while perception of unpleasantness is more responsive in the posterior ACC [79]. The ACC sends affective and motivational outputs to the striatum, PFC, hypothalamus and amygdala [68]. Some affective and motivational signals are incorporated with signals from the motor cortex in the caudal putamen, which participates in the sensorimotor circuit of the basal ganglia [14]. The other signals from the ACC cooperates with signals from the PFC in the rostral putamen and nucleus accumbens, and they participate in the associative and limbic circuits of the basal ganglia [68]. Signals sent to the PFC, hypothalamus, and amygdala are associated with the activation of motivational functions related to pain.
The PFC is a region which has the opioid receptors, which is the center of motivational aspect of pain such as predicting the onset of pain, avoiding pain, and generating will against pain [3,68]. Alcoholic behavior, drug seeking behavior, or binge-type eating disorder can be seen in dependent of activity of the PFC [3]. It participates in emotional regulation of the basal ganglia via connection with the nucleus accombens, which evokes involuntary motivation related to avoidance of pain [4]. The PFC projects a large amount of fibers to the hypothalamus and to the amygdala via the medial forebrain bundle and uncinated fasciculus, respectively [27].
The hypothalamus (Ⓘ in Fig. 1) is the control center of the autonomic nervous system. It receives affective and motivational information related to pain from the ACC and PFC, and it activates the autonomic nervous system to support execution of motivations by changing heart rate, respiration, muscle tension or bowel movement [27]. The hypothalamus also receives noxious information from the PBN, and has reciprocal connection with the amygdala [16,26,27]. Efferent fibers direct to the ventral tegmental area (VTA), which in turn modulates the basal ganglia and thalamus, and to the PAG, which plays as the emotional motor system as addressed above [74,77,78].
The amygdala (Ⓙ in Fig. 1) receives massive affective and motivational inputs from the PFC and ACC, and nociceptive inputs from the PBN as the hypothalamus does. When this information cooperatively activates the amygdala, it may evoke fear, aggression, anxiety or stress that related to pain [16,55]. The amygdala has reciprocal connections with the PAG and hypothalamus, and sends output signals to the locus coeruleus. The locus coeruleus releases noradrenergic transmission to the trigeminal nucleus caudalis, which induces antinociception as a part of the descending pain modulation system [55,78].
There are also motor responses responding to pain sensation other than the reflex of the autonomic nervous system. The motor cortex (Ⓚ in Fig. 1) receives noxious information from the CM/PF. The pyramidal neuron of the motor cortex not only mobilizes voluntary motor system through the corticospinal tract but also releases collateral projections to the sensorimotor region of the striatum, i.e., the putamen. This collateral projection may facilitate the sensorimotor circuit of the basal ganglia, so that an extrapyramidal symptom of the sensorimotor circuit may appear [66]. Affective components of pain signal from the ACC that synapses in the putamen forms an integrative signal of the affective and sensorimotor aspects of pain [14]. The integrative signal from the putamen is delivered via the globus pallidus internus to the thalamic recipients, i.e., the ventral anterior, ventral lateral nuclei and CM/PF. In the CM/PF, the integrative sensorimotor signal participates in the processing of nociception, pain response and polysensory integration in general, and engages in the overall motor output related to pain [33].
Following the aforementioned background, ready to discuss how to build a strategy of neuromodulation for chronic trigeminal neuropathy. As pain is a complex phenomenon that is subjectively experienced through the collaboration of related brain networks, neuromodulation techniques with a single target may not be enough for adequate pain control [59]. When severe facial pain persists even after exhausting all available conventional therapeutic methods, neuromodulation could provide a last resort for these patients. Although past clinical experiences showed weak evidence on neuromodulation as an effective option for chronic trigeminal neuropathy, as the mechanisms of analgesic stimulation are yet to be clearly delineated, new knowledge may pave the way for better neuromodulation in the future [58].
The VPM nucleus of thalamus (Ⓑ in Fig. 1) has long been considered as a preferred target for intractable trigeminal neuropathy due to the following reasons [30,43,47,49]. First, the VPM relays somatosensory information from the face via the spinothalamic and lemniscal pathways to the primary somatosensory cortex. Second, since the 1970s, it has been known that paresthesia evoked by an electrical stimulation may have an analgesic effect [6,12,30,47,62]. Third, the gate-control theory, a very popular ideological theory, addressed that facilitation of a large-diameter fiber could suppress propagation of pain and temperature signals conducted via small-diameter fibers [50].
The PAG (Ⓓ in Fig. 1) was firstly introduced by Richardson and Akil [63] as another target for analgesic DBS in the 1970s. Then Hosobuchi et al. [31] reported that analgesic effect produced by electrical stimulations of the PAG leads to the production of the endorphin in this region. The PAG is considered to be a hub of the descending pain-modulation system, so it has long been considered another principal target for intractable trigeminal pain [2,6,13,43,71].
Several studies utilized both targets for neuromodulation of chronic trigeminal neuropathy, and early evidence of DBS at the VPM and the PAG showed favorable results for those patie nts [2,6,30,31,38,44,49,63,70,85]. It was reported that long-term success rates, i.e., more than 50% of pain reduction perceived by patients, were varied ranging from 0% to 83% [59]. Nevertheless, most of these studies concluded that DBS of the PAG and the VPM may be effective in treatment of chronic intractable pain syndromes. Among those, chronic trigeminal neuropathy was reported with relatively better results than the other pain syndromes [29,54].
However, the phenomenon of tolerance that indicates reduction of initial therapeutic effect over time was consistently reported [44]. In 2001, Coffey [19] reported results of two multicenter trials conducted by the device manufacturer, of which the original purpose was acquisition of approval from the US Food and Drug Administration. However, it had several aspects that were inappropriate for scientific literature. The independent variable was commercial models produced by the manufacturer rather than disease categories or stimulation targets. Institutions participated in this trial were too heterogeneous that made the treatment outcome unreliable. Moreover, important clinical information such as DBS targets stimulated, the number of electrodes, and the parameters were not defined [18,58]. This study reported that percentages of patients with more than 50% pain relief were 46.1% and 17.8% at 12 and 24 months, for the model 3380, and 16.2% and 13.5% at 12 and 24 months, for the model 3387, respectively. Based on the observation, the author concluded that analgesic DBS had no benefits. Unfortunately, this unsubstantial conclusion seems to significantly discourage following investigators trying to study brain stimulation for pain control.
The first MCS (Ⓚ in Fig. 1) was reported by Tsubokawa et al. [76] in 1991. Twelve patients with deafferentational pain underwent MCS, and nine of them presented with excellent or good pain reduction after the MCS. From the point of view of the gate-control theory by Melzack and Wall [50], it should be the sensory cortex stimulation that would bring analgesic effects by the activation of the large-fiber somatosensory system. However, stimulation of the sensory cortex had no analgesic effect, and thalamic hypersensitivity was found to be inhibited not by the sensory cortex stimulation but by the MCS [76]. Following studies also indicated that the MCS appeared to hold a promising result for patients with chronic trigeminal neuropathy, deafferentational pain, and other forms of intractable pain that failed to respond to conventional therapeutic strategies [12,29,43,60]. A recent double-blinded randomized study for MCS for chronic neuropathic pain showed that modest but significant pain reduction differences between sham and active stimulation of the MCS [29]. This study showed that analgesic effects of the MCS depended on the presence of the placebo effect. Moreover, there are concerns that the MCS also lacks long-term follow-up data on the effectiveness for pain control [12].
Those three types of neuromodulation technique, i.e., VPM DBS, PAG DBS, and MCS, showed promising clinical results in studies of early periods. However, such enthusiastic outcomes were not supported by following studies bearing well-designed and long-term follow-up data. These three targets have a common limitation that they just focused on the control of the sensory component of pain. As pain has affective, emotional, and sensory components, neuromodulation for analgesia would not be successful only by modulating the sensory component of pain. Therefore, the success of neuromodulation for analgesia might depend on appropriately influencing the pathways integral to the various components of pain perception.
Nucleus accumbens is situated at an anterior ventral aspect of the striatum, which is responsible for reward-seeking behavior, motivation, and emotion [4,74]. It receives glutamatergic inputs from the PFC, CM/PF, amygdala, and hippocampus, and receives dopaminergic afferents from the VTA [56,74]. For this reason, the nucleus accombens is also believed to be engaged in the emotional process of pain control. A case of nucleus accombens stimulation for analgesia was reported, which brought additional pain reduction along with periventricular gray matter (PVG) stimulation [46]. However, DBS of the nucleus accombens for pain control was not supported by clinical studies. The nucleus accumbens is a highly complex and interdigitated structure of relatively large volume; therefore, it may be difficult to find the best location for pain control in the nucleus accumbens.
As discussed above, the CM/PF (Ⓕ in Fig. 1) mediates polysensory, motor, emotional, and affective information at the center of the brain, so that CM/PF DBS possibly controls sensory, affective, and motivational aspects of pain [33]. However, it should not be ignored that the CM/PF also has very complex and well-stratified regions each responsible for sensorimotor, associative, and limbic functions of the brain [72,79,82]. This complexity might restrict consistent clinical effects expected by the CM/PF DBS. Interestingly, anatomical location of the CM/PF is closely related to the PAG and PVG [59]. The CM/PF is placed just lateral to the PVG, and is situated in the passage of the electrode directing to the PAG [11,38,71]. This anatomical proximity to the PAG and PVG renders a question on whether it is possible that the analgesic effects of PAG or PVG DBS is partially induced by the leakage of electrical energy to the CM/PF [37]. Recently, analgesic DBS of the CM/PF showed sustained improvement of chronic neuropathic pain in long-term observations of >4 years [1]. The best stimulation effects were obtained in patients with facial pain, brachial plexus injury, or central pain. Furthermore, anatomical connections and the neurophysiological role of the CM/PF make strong suggestion that CM/PF DBS may be a reasonable alternative for chronic trigeminal neuropathies [82].
The authors of the present study believe that the most underestimated targets of DBS for analgesia are the STN and GPi. STN stimulation is known to modulate the GPi, substantia nigra, motor cortex and non-motor limbic circuits. The STN DBS has long been applied to alleviate movement disorders, especially Parkinson’s disease. In Parkinson’s disease, the principal purpose of the STN DBS is to control motor symptoms. Meanwhile pain, which is a common feature accompanied by parkinsonian motor symptoms, was also shown to be improved by the STN DBS in several studies [36,48]. This analgesic effect was not merely a consequence of the improvement of motor symptoms but an independent phenomenon related to a central modulation of pain threshold indicating that the STN DBS may disrupt pathologic sensory-signal transduction [36,48]. This is supported by the observation that STN and GPi DBS increased blood oxygenation levels in the motor cortex, cingulate cortex, and sensorimotor cortex in a frequency-dependent fashion [39].
Similarly, the GPi DBS has been shown to have analgesic effects. The GPi has been used as the primary target of stereotactic surgery for the treatment of dystonia and dyskinesia [28,53,65]. As the GPi receives afferents from the striatum and the STN, and projects efferents to the CM/PF, the ventral anterior/posterior nuclei, and the pedunculopontine nucleus, the GPi DBS may affect brain networks using these afferent and efferent fibers [39]. Among those, connections with the STN, striatum and CM/PF could participate in the modulation of pain perception. In the studies addressing clinical effects of the GPi DBS on dystonia patients, pain reduction was consistently reported after pallidal stimulation [33,56]. Interestingly, analgesic effect of the GPi DBS showed a tolerance indicating a partial loss of initial pain reduction in long-term follow-up, while the anti-dystonic effects had a delayed benefit, i.e., a progressive increase in the therapeutic benefits in dystonia [17,32]. These mutually conflicting clinical course means that the therapeutic effects of the GPi DBS for pain and dystonia are achieved through different mechanisms in the brain [32].
The ACC (Ⓗ in Fig. 1) used to be recognized as a target of lesioning procedure for intractable pain [12]. The ACC is located dorsally to the genu portion of corpus callosum on the medial surface of the brain, where it is relatively shallow from the cranial vault, compared to the other DBS targets [79]. Surface area of the ACC seems to be quite large as a single target for analgesic DBS. DBS of the ACC was applied for the treatment of refractory thalamic pain syndrome, which showed 35% of pain reduction at 18 months follow-up [42]. Levi et al. [42] reported that ACC DBS for intractable chronic pain significantly improved scores of quality of life and physical functioning domain. To our knowledge, there is no study addressing ACC DBS for chronic trigeminal neuropathy. Nevertheless, the ACC is a higher center of the affective component of pain and has a role in mediating emotional aspect of pain, so that ACC stimulation might bear a potential for alleviation of intractable pain.
Numerous studies reported that DBS of the hypothalamus (Ⓘ in Fig. 1) was effective for treating cluster headaches, a major type of TAC [41,43,80,81]. The hypothalamus is the central hub for control of the autonomic nervous system, which is believed to play an essential role in the pathophysiology of cluster headache [80]. Although the DBS at the VTA was also reported for the treatment of cluster headache, its effect may rather be arising from stimulation of the hypothalamus than from the VTA, due to the anatomical proximity between the VTA and hypothalamus [15]. As the hypothalamus receives neural signals from the ACC, PFC and PBN, and relays to the PAG, amygdala, and trigeminal nucleus caudalis, the hypothalamic DBS is applied for other craniofacial pain syndromes, like migraine and trigeminal neuropathy [41,81].
The sphenopalatine ganglion is a large parasympathetic ganglion located at pterygopalatine fossa [40,84]. Post-ganglionic parasympathetic fibers innervate several craniofacial structures, including the lacrimal gland, nasal gland, meningeal vessels and cerebral blood vessels [40]. Other nerve fibers, such as sympathetic fibers and maxillary division of trigeminal nerve, also encompass this ganglion [40,84]. The sphenopalatine ganglion has been targeted for neuromodulation of cluster headache because it relays parasympathetic signal that is activated in paroxysmal attacks in trigeminal autonomic neuropathies. Several reports indicated that patients with sphenopalatine ganglion stimulation received pain relief in both short and long-term studies [67].
The occipital nerve, comprising of the greater and lesser occipital nerves, was stimulated to modulate several types of medically intractable craniofacial pain syndromes, including migraine, occipital neuralgia, cervicogenic headache, cluster headache, and chronic trigeminal neuropathy [10,23,34,52,80,81]. As the occipital nerve is directed to the trigeminal nucleus caudalis, occipital nerve stimulation may modulate neural activity of the primary sensory neuron of the craniofacial area [10]. Therefore, occipital nerve stimulation may alleviate not only occipital area pain but also craniofacial pain [80]. Occipital nerve stimulation showed significant clinical benefit for migraine, tension type headache, and cluster headache in long-term prospective cohort studies [80]. A multicenter randomized study of occipital nerve stimulation for migraine indicated that about 40% of patients with migraine achieved appropriate headache relief [80]. Consequently, when conventional therapeutic strategies fail, occipital nerve stimulation could be considered for chronic trigeminal neuropathy.
Neuromodulation interventions for trigeminal neuralgia are yet to be elucidated. Even though much research has been conducted on this challenge for more than 40 years, a significant volume of facial pain syndrome still remains truly intractable. However, recent developments in the field of pain processing and clinical data will endow new hope for the conquest of chronic trigeminal neuropathy.
Notes
Conflicts of interest
Moonyoung Chung has been editorial board of JKNS since November 2017. He was not involved in the review process of this article. No potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant, funded by the Korean Ministry of Science and ICT (No. 2019R1G1A1100439). This work was also supported by the Soonchunhyang University Research Fund.
References
1. Abdallat M, Saryyeva A, Blahak C, Wolf ME, Weigel R, Loher TJ, et al. Centromedian-parafascicular and somatosensory thalamic deep brain stimulation for treatment of chronic neuropathic pain: a contemporary series of 40 patients. Biomedicines. 9:731. 2021.
2. Adams JE. Naloxone reversal of analgesia produced by brain stimulation in the human. Pain. 2:161–166. 1976.
3. Baldo BA. Prefrontal cortical opioids and dysregulated motivation: a network hypothesis. Trends Neurosci. 39:366–377. 2016.
4. Bálint E, Csillag A. Nucleus accumbens subregions: hodological and immunohistochemical study in the domestic chick (gallus domesticus). Cell Tissue Res. 327:221–230. 2007.
5. Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 139:267–284. 2009.
6. Basbaum AI, Fields HL. Endogenous pain control mechanisms: review and hypothesis. Ann Neurol. 4:451–462. 1978.
7. Ben-Haim S, Mirzadeh Z, Rosenberg WS. Deep brain stimulation for intractable neuropathic facial pain. Neurosurg Focus. 45:E15. 2018.
8. Bendtsen L, Zakrzewska JM, Heinskou TB, Hodaie M, Leal PRL, Nurmikko T, et al. Advances in diagnosis, classification, pathophysiology, and management of trigeminal neuralgia. Lancet Neurol. 19:784–796. 2020.
9. Berridge KC, Kringelbach ML. Pleasure systems in the brain. Neuron. 86:646–664. 2015.
10. Blake P, Burstein R. Emerging evidence of occipital nerve compression in unremitting head and neck pain. J Headache Pain. 20:76. 2019.
11. Boivie J, Meyerson BA. A correlative anatomical and clinical study of pain suppression by deep brain stimulation. Pain. 13:113–126. 1982.
12. Burchiel KJ, Raslan AM. Contemporary concepts of pain surgery. J Neurosurg. 130:1039–1049. 2019.
13. Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci. 14:502–511. 2013.
14. Calzavara R, Mailly P, Haber SN. Relationship between the corticostriatal terminals from areas 9 and 46, and those from area 8a, dorsal and rostral premotor cortex and area 24c: an anatomical substrate for cognition to action. Eur J Neurosci. 26:2005–2024. 2007.
15. Cappon D, Ryterska A, Lagrata S, Miller S, Akram H, Hyam J, et al. Ventral tegmental area deep brain stimulation for chronic cluster headache: effects on cognition, mood, pain report behaviour and quality of life. Cephalalgia. 39:1099–1110. 2019.
16. Chiang MC, Bowen A, Schier LA, Tupone D, Uddin O, Heinricher MM. Parabrachial complex: a hub for pain and aversion. J Neurosci. 39:8225–8230. 2019.
17. Chung M, Huh R. Different clinical course of pallidal deep brain stimulation for phasic- and tonic-type cervical dystonia. Acta Neurochir (Wien). 158:171–180. 2016.
18. Clelland CD, Zheng Z, Kim W, Bari A, Pouratian N. Common cerebral networks associated with distinct deep brain stimulation targets for cluster headache. Cephalalgia. 34:224–230. 2014.
19. Coffey RJ. Deep brain stimulation for chronic pain: results of two multicenter trials and a structured review. Pain Med. 2:183–192. 2001.
20. Cruccu G, Di Stefano G, Truini A. Trigeminal neuralgia. N Engl J Med. 383:754–762. 2020.
21. Ebel H, Rust D, Tronnier V, Böker D, Kunze S. Chronic precentral stimulation in trigeminal neuropathic pain. Acta Neurochir (Wien). 138:1300–1306. 1996.
22. Ferbinteanu J. Memory systems 2018 - towards a new paradigm. Neurobiol Learn Mem. 157:61–78. 2019.
23. Fontaine D, Bozzolo E, Chivoret N, Paquis P, Lanteri-Minet M. Salvage treatment of trigeminal neuralgia by occipital nerve stimulation. Cephalalgia. 34:307–310. 2014.
24. French JD, Verzeano M, Magoun HW. An extralemniscal sensory system in the brain. AMA Arch Neurol Psychiatry. 69:505–518. 1953.
25. Fuller PM, Sherman D, Pedersen NP, Saper CB, Lu J. Reassessment of the structural basis of the ascending arousal system. J Comp Neurol. 519:933–956. 2011.
26. Godfrey N, Borgland SL. Diversity in the lateral hypothalamic input to the ventral tegmental area. Neuropharmacology. 154:4–12. 2019.
27. Gouveia FV, Hamani C, Fonoff ET, Brentani H, Alho EJL, De Morais RMCB, et al. Amygdala and hypothalamus: historical overview with focus on aggression. Neurosurgery. 85:11–30. 2019.
28. Gruber D, Südmeyer M, Deuschl G, Falk D, Krauss JK, Mueller J, et al. Neurostimulation in tardive dystonia/dyskinesia: a delayed start, sham stimulation-controlled randomized trial. Brain Stimul. 11:1368–1377. 2018.
29. Hamani C, Fonoff ET, Parravano DC, Silva VA, Galhardoni R, Monaco BA, et al. Motor cortex stimulation for chronic neuropathic pain: results of a double-blind randomized study. Brain. 144:2994–3004. 2021.
30. Hosobuchi Y, Adams JE, Rutkin B. Chronic thalamic stimulation for the control of facial anesthesia dolorosa. Arch Neurol. 29:158–161. 1973.
31. Hosobuchi Y, Rossier J, Bloom FE, Guillemin R. Stimulation of human periaqueductal gray for pain relief increases immunoreactive beta-endorphin in ventricular fluid. Science. 203:279–281. 1979.
32. Huh R, Song IU, Chung M. Neuropsychological consequences of pallidal deep brain stimulation altering brain networks. J Clin Neurosci. 54:50–56. 2018.
33. Ilyas A, Pizarro D, Romeo AK, Riley KO, Pati S. The centromedian nucleus: anatomy, physiology, and clinical implications. J Clin Neurosci. 63:1–7. 2019.
34. Keifer OP Jr, Diaz A, Campbell M, Bezchlibnyk YB, Boulis NM. Occipital nerve stimulation for the treatment of refractory occipital neuralgia: a case series. World Neurosurg. 105:599–604. 2017.
35. Khan S, Schoenen J, Ashina M. Sphenopalatine ganglion neuromodulation in migraine: what is the rationale? Cephalalgia. 34:382–391. 2014.
36. Kim HJ, Paek SH, Kim JY, Lee JY, Lim YH, Kim MR, et al. Chronic subthalamic deep brain stimulation improves pain in parkinson disease. J Neurol. 255:1889–1894. 2008.
37. Krüger MT, Avecillas-Chasin JM, Heran MKS, Naseri Y, Sandhu MK, Polyhronopoulos NE, et al. Directional deep brain stimulation can target the thalamic "sweet spot" for improving neuropathic dental pain. Oper Neurosurg (Hagerstown). 21:81–86. 2021.
38. Kumar K, Toth C, Nath RK. Deep brain stimulation for intractable pain: a 15-year experience. Neurosurgery. 40:736–746. 1997.
39. Lai HY, Younce JR, Albaugh DL, Kao YC, Shih YY. Functional MRI reveals frequency-dependent responses during deep brain stimulation at the subthalamic nucleus or internal globus pallidus. Neuroimage. 84:11–18. 2014.
40. Láinez MJ, Marti AS. Sphenopalatine ganglion stimulation in cluster headache and other types of headache. Cephalalgia. 36:1149–1155. 2016.
41. Leone M, Proietti Cecchini A. Deep brain stimulation in headache. Cephalalgia. 36:1143–1148. 2016.
42. Levi V, Cordella R, D'Ammando A, Tringali G, Dones I, Messina G, et al. Dorsal anterior cingulate cortex (ACC) deep brain stimulation (DBS): a promising surgical option for the treatment of refractory thalamic pain syndrome (TPS). Acta Neurochir (Wien). 161:1579–1588. 2019.
43. Levy R, Deer TR, Henderson J. Intracranial neurostimulation for pain control: a review. Pain Physician. 13:157–165. 2010.
44. Levy RM, Lamb S, Adams JE. Treatment of chronic pain by deep brain stimulation: long term follow-up and review of the literature. Neurosurgery. 21:885–893. 1987.
45. Maarbjerg S, Di Stefano G, Bendtsen L, Cruccu G. Trigeminal neuralgia - diagnosis and treatment. Cephalalgia. 37:648–657. 2017.
46. Mallory GW, Abulseoud O, Hwang SC, Gorman DA, Stead SM, Klassen BT, et al. The nucleus accumbens as a potential target for central poststroke pain. Mayo Clin Proc. 87:1025–1031. 2012.
47. Marchand S, Kupers RC, Bushnell CM, Duncan GH. Analgesic and placebo effects of thalamic stimulation. Pain. 105:481–488. 2003.
48. Marques A, Chassin O, Morand D, Pereira B, Debilly B, Derost P, et al. Central pain modulation after subthalamic nucleus stimulation: a crossover randomized trial. Neurology. 81:633–640. 2013.
49. Mazars GJ. Intermittent stimulation of nucleus ventralis posterolateralis for intractable pain. Surg Neurol. 4:93–95. 1975.
50. Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 150:971–979. 1965.
51. Meyerson BA, Lindblom U, Linderoth B, Lind G, Herregodts P. Motor cortex stimulation as treatment of trigeminal neuropathic pain. Acta Neurochir Suppl (Wien). 58:150–153. 1993.
52. Miller S, Watkins L, Matharu M. Treatment of intractable chronic cluster headache by occipital nerve stimulation: a cohort of 51 patients. Eur J Neurol. 24:381–390. 2017.
53. Moro E, Piboolnurak P, Arenovich T, Hung SW, Poon YY, Lozano AM. Pallidal stimulation in cervical dystonia: clinical implications of acute changes in stimulation parameters. Eur J Neurol. 16:506–512. 2009.
54. Nandi D, Aziz T, Carter H, Stein J. Thalamic field potentials in chronic central pain treated by periventricular gray stimulation -- a series of eight cases. Pain. 101:97–107. 2003.
55. Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. J Clin Invest. 120:3779–3787. 2010.
56. Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev. 20:91–127. 1995.
57. Parmar M, Sharma N, Modgill V, Naidu P. Comparative evaluation of surgical procedures for trigeminal neuralgia. J Maxillofac Oral Surg. 12:400–409. 2013.
58. Pereira EA, Aziz TZ. Neuropathic pain and deep brain stimulation. Neurotherapeutics. 11:496–507. 2014.
59. Pereira EA, Green AL, Aziz TZ. Deep brain stimulation for pain. Handb Clin Neurol. 116:277–294. 2013.
60. Rapisarda A, Ioannoni E, Izzo A, Montano N. What are the results and the prognostic factors of motor cortex stimulation in patients with facial pain? A systematic review of the literature. Eur Neurol. 84:151–156. 2021.
61. Rasskazoff SY, Slavin KV. Neuromodulation for cephalgias. Surg Neurol Int. 4:S136–S150. 2013.
62. Reynolds DV. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 164:444–445. 1969.
63. Richardson DE, Akil H. Pain reduction by electrical brain stimulation in man. Part 1: acute administration in periaqueductal and periventricular sites. J Neurosurg. 47:178–183. 1977.
64. Sadikot AF, Rymar VV. The primate centromedian-parafascicular complex: anatomical organization with a note on neuromodulation. Brain Res Bull. 78:122–130. 2009.
65. Schjerling L, Hjermind LE, Jespersen B, Madsen FF, Brennum J, Jensen SR, et al. A randomized double-blind crossover trial comparing subthalamic and pallidal deep brain stimulation for dystonia. J Neurosurg. 119:1537–1545. 2013.
66. Schmahmann JD, Pandya DN. Disconnection syndromes of basal ganglia, thalamus, and cerebrocerebellar systems. Cortex. 44:1037–1066. 2008.
67. Schoenen J, Jensen RH, Lantéri-Minet M, Láinez MJ, Gaul C, Goodman AM, et al. Stimulation of the sphenopalatine ganglion (SPG) for cluster headache treatment. Pathway CH-1: a randomized, sham-controlled study. Cephalalgia. 33:816–830. 2013.
68. Sewards TV, Sewards MA. The medial pain system: neural representations of the motivational aspect of pain. Brain Res Bull. 59:163–180. 2002.
69. Shephard MK, Macgregor EA, Zakrzewska JM. Orofacial pain: a guide for the headache physician. Headache. 54:22–39. 2014.
70. Shulman R, Turnbull IM, Diewold P. Psychiatric aspects of thalamic stimulation for neuropathic pain. Pain. 13:127–135. 1982.
71. Sims-Williams HP, Javed S, Pickering AE, Patel NK. Characterising the analgesic effect of different targets for deep brain stimulation in trigeminal anaesthesia dolorosa. Stereotact Funct Neurosurg. 94:174–181. 2016.
72. Smith Y, Raju DV, Pare JF, Sidibe M. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci. 27:520–527. 2004.
73. Spina A, Mortini P, Alemanno F, Houdayer E, Iannaccone S. Trigeminal neuralgia: toward a multimodal approach. World Neurosurg. 103:220–230. 2017.
74. Stuber GD, Britt JP, Bonci A. Optogenetic modulation of neural circuits that underlie reward seeking. Biol Psychiatry. 71:1061–1067. 2012.
75. Tepper SJ, Stillman MJ. Cluster headache: potential options for medically refractory patients (when all else fails). Headache. 53:1183–1190. 2013.
76. Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S. Chronic motor cortex stimulation for the treatment of central pain. Acta Neurochir Suppl (Wien). 52:137–139. 1991.
77. Varela C. Thalamic neuromodulation and its implications for executive networks. Front Neural Circuits. 8:69. 2014.
78. Venkatraman A, Edlow BL, Immordino-Yang MH. The brainstem in emotion: a review. Front Neuroanat. 11:15. 2017.
79. Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 6:533–544. 2005.
80. Vukovic Cvetkovic V, Jensen RH. Neurostimulation for the treatment of chronic migraine and cluster headache. Acta Neurol Scand. 139:4–17. 2019.
81. Weber K. Neuromodulation and devices in trigeminal neuralgia. Headache. 57:1648–1653. 2017.
82. Weigel R, Krauss JK. Center median-parafascicular complex and pain control. Review from a neurosurgical perspective. Stereotact Funct Neurosurg. 82:115–126. 2004.
83. Wiech K. Deconstructing the sensation of pain: the influence of cognitive processes on pain perception. Science. 354:584–587. 2016.
84. William A, Azad TD, Brecher E, Cherry T, Bernstein I, Bruce DM, et al. Trigeminal and sphenopalatine ganglion stimulation for intractable craniofacial pain--case series and literature review. Acta Neurochir (Wien). 158:513–520. 2016.
85. Young RF, Kroening R, Fulton W, Feldman RA, Chambi I. Electrical stimulation of the brain in treatment of chronic pain. Experience over 5 years. J Neurosurg. 62:389–396. 1985.
Fig. 1.
Nociceptive networks related to the trigeminal nucleus. Nociception from the trigeminal nerve enters the central nervous system via trigeminal nucleus caudalis (Ⓐ). This primary sensory nucleus sends nociceptive information to the ventral posterior medial nucleus (VPL, Ⓑ), parabrachial nucleus (PBN, Ⓒ), periaqueductal gray (PAG, Ⓓ), and the centromedian (CM)/parafascicular (PF) nucleus (Ⓕ). The CM/PF projects glutamatergic fibers to the striatum (Ⓖ), and relays polysensory integrative information throughout the entire cortices, especially to the anterior cingulate cortex (Ⓗ), insular cortex, prefrontal cortex, primary sensory cortex, and motor cortex. The anterior cingulate cortex and the prefrontal cortex release efferent to the hypothalamus (Ⓘ) and the amygdala (Ⓙ), which processes affective and emotional aspects of pain. The motor cortex (Ⓚ) projects output signal to the subthalamic nucleus (STN) and the striatum, involving procedural memory formation of the basal ganglia. Output signal from the basal ganglia is relayed via the substantia nigra pars reticulata (SNr) and the globus pallidus internus (GPi) to the thalamic recipient. The hypothalamus regulates the autonomic nervous system via the ventral tegmental area (VTA) and the PAG. The PAG releases descending signals for pain modulation (broken arrows) to the nucleus raphe magnus (NRM, Ⓔ), which in turn modulates the trigeminal nucleus caudalis. The amygdala also participates descending pain modulation via locus coeruleus (LC) to the trigeminal nucleus caudalis. VPM : ventral posterior medial.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download