INTRODUCTION
MATERIALS AND METHODS
Study population
Table 1.
Endovascular procedures using the frontier-wire technique
Application of the rigid-tipped guidewire
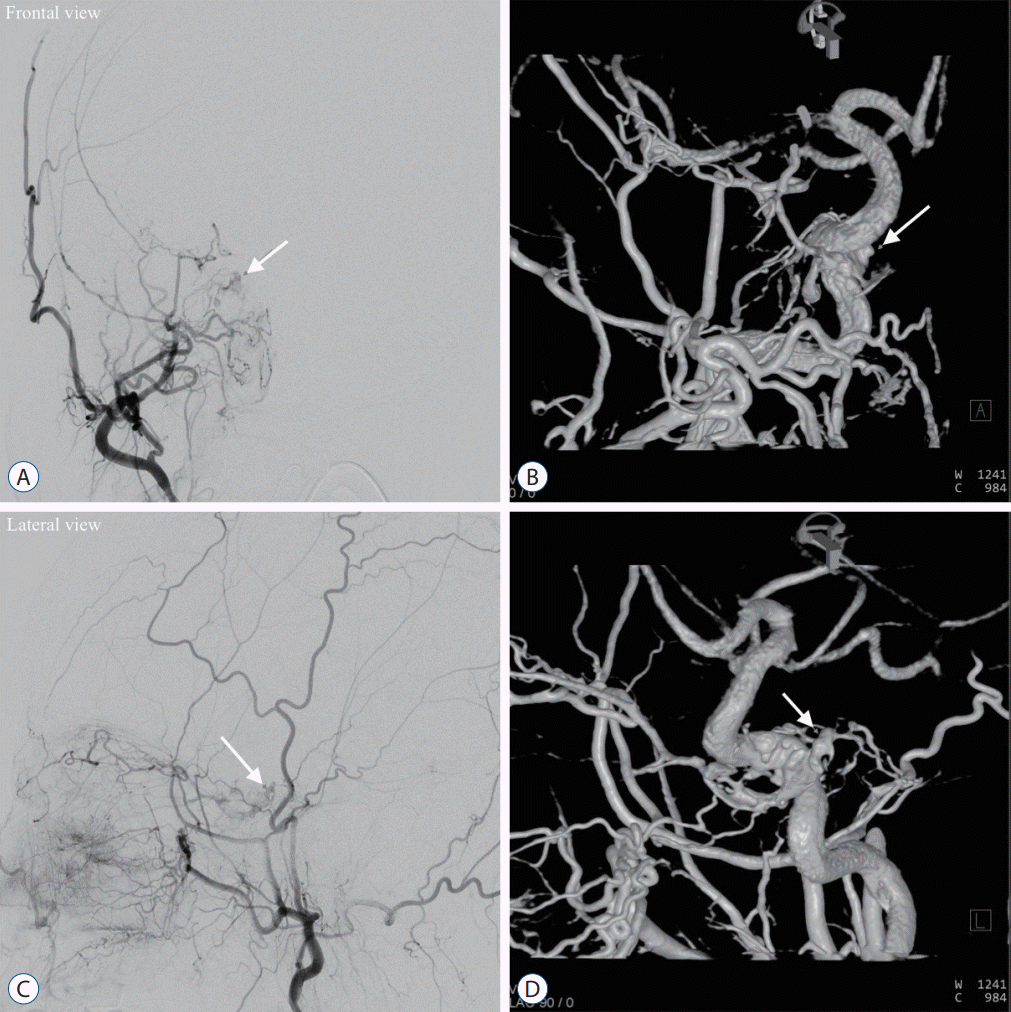 | Fig. 1.Showing illustration of the case (case 4). The patient complained of the right conjunctival injection and sixth cranial nerve palsy. Right cavernous sinus dural arteriovenous fistula (CSDAVF) was demonstrated in both frontal (A and B) and lateral views (C and D) of the right external carotid artery (ECA) angiography and 3D rotational angiography. The 3D rotational angiography of the right ECA was contaminated by the ipsilateral ICA opacification. Ipsilateral superior petrosal sinus, inferior petrosal sinus, and intercavernous sinus were occluded. Venous drainage via a mainly ipsilateral superior ophthalmic vein and partly inferior ophthalmic vein were noted. The white solid arrow in each panel (A-D) indicated the shunted pouch (SP). Multiple dural feeders fed the SP from the bilateral internal carotid artery and ECA, such as a middle meningeal artery, accessory meningeal artery, ascending pharyngeal artery, artery of foramen Rotundum, and meningohypophyseal trunk. |
Evaluations and analyses
RESULTS
 | Fig. 2.Showing intraoperative procedures (case 4). A : A 4-F diagnostic catheter was placed at the right proximal external carotid artery (ECA) for the arteriographic roadmap. A 6-F guide catheter was introduced through the right internal jugular vein. The 6-F guide catheter and another 4-F diagnostic catheter were used by means of the coaxial technique. To reinforce the 0.035-in guidewire, the 6-F guide catheter was inserted as close as possible to the right inferior petrosal sinus (IPS) orifice. The 4-F diagnostic catheter was also inserted into the distal part of the right IPS. Performing the frontier-wire technique, arteriographic and venographic roadmaps were acquired simultaneously with the presence of the 0.035-in guidewire. The microcatheter was successfully advanced with the guidance of the white footprint of the guidewire (white arrows). The microcatheter tip seemed to be in the shunted pouch (SP) (white arrowheads). B : Control angiogram, the microcatheter tip was in the isolated posterior aspect of the right cavernous sinus (CS) (white arrow). The membranous barrier could not be overcome by the neurointerventional microguidewires or 0.035-in guidewire. C : Under the biplane right ECA roadmaps, the direction of the microcatheter tip was controlled precisely using the neurointerventional microguidewire. With the removal of the neurointerventional microguidewire, the rigid-tipped microguidewire was introduced gently and then inserted only 1 mm further than the microcatheter tip (white arrows). Holding firmly the rigid-tipped microguidewire, the microcatheter could be advanced beyond the obstacle under the guidance of the rigid-tipped microguidewire. D : After removing the rigid-tipped microguidewire, control angiogram showed that the microcatheter tip was in the SP (white arrowhead). E : The microcatheter was able to navigate the CS using the microguidewire. Subsequent coil embolization of both the entry of the venous drainage and SP was done. The white arrow indicated the coil mass packed in the SP. In the final right ECA arteriography, the cavernous sinus dural arteriovenous fistula was obliterated and no residual flow. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download