Abstract
Sialodochitis fibrinosa is a disease characterized by salivary duct obstruction. A 21-year-old male presented with a painful submandibular gland (SMG) swelling. Serum eosinophilia and Whartons’ duct dilatation with strong enhancement were observed on contrast CT. Core needle biopsy (CNB) for SMG parenchyma revealed lymphocytic infiltration between dilated intralobular ducts. In another case, a 39-year-old male complained of recurrent major salivary glands swelling for ten years with an itching sensation on the overlying skin of the salivary gland. Enhancement of both SMG parenchyma, dilatation of both Wharton’s ducts and elevated serum eosinophilia were observed on contrast CT study. CNB for SMG parenchyma revealed lymphocytic infiltration with many eosinophils around a markedly dilated interlobular duct. The recurrent SMG swelling in both cases were relieved by antihistamine medication, warranting suspicion that these cases might organ-specific eosinophilic disease. We dicuss these two cases with a literature review.
Etiologies of recurrent inflammation of the salivary gland vary. Among various etiologies of this condition, eosinophil infiltration in the salivary gland parenchyma is one of possible causes of recurrent inflammation. This has been understood as a form of organ-specific eosinophilic disease [1]. Sialodochitis fibrinosa was first reported by Kussmaul in 1879. It is a disease characterized by salivary duct obstruction due to mucofibrinous plugs, resulting in obstructive symptoms of salivary glands [1,2]. This disease has also recently been called “eosinophilic sialodochitis.” Although diagnostic criteria for eosinophilic sialodochitis have not been well established, specific findings commonly observed in this disease can aid its diagnosis [3]. In addition, antihistamine medication usually improves obstructive symptoms in this disease [4]. Herein, we report two cases of recurrent submandibular gland (SMG) swelling relieved by antihistamine medication, suspicious of organ-specific eosinophilic disease, with a literature review.
A 21-year-old male presented with painful parotid and SMG swelling for the past 10 days. Although his parotid pain subsided after taking nonsteroidal antiinflammatory drug, painful SMG swelling continued until presentation. He was healthy without a history of any disease including allergic or metabolic disease. On physical examination, both SMGs were enlarged without a notable enlargement of lymph node. Contrast CT revealed that both Wharton’s ducts were dilated, showing a strong enhancement along with the duct (Fig. 1A). Under suspicion of acute sialadenitis, antibiotics (cefditoren 100 mg three times per day for 7 days) and prednisolone (5 mg twice a day for 5 days) were prescribed. We encouraged the patient to have frequent hydration and SMG massage for facilitating drainage of saliva. SMG swelling and pain subsided one week later. Since he wanted to know the cause of disease, laboratory tests for diabetes mellitus, autoimmune disease (anti-SS-A/Ro Ab, anti-SS-B/La Ab, rheumatoid factor [RF], antinuclear Ab [ANA], antineutrophil cytoplasmic Ab, complement component 3 and 4, immunoglobulin G [IgG]), and thyroid function were performed. Only serum eosinophilia showed a high level at 6.8% (normal range: 0%-5%). Other results were within normal limits. We again recommended frequent hydration and salivary gland massage without medication. During the following week, he complained of gradually progressive swelling and pain in both SMGs. We additionally checked multiple antigen simultaneous test. Only elevated total IgE at 403.4 IU/mL (normal limit: 100 IU/mL) was found. We performed ultrasonography (US) followed by core needle biopsy (CNB) for SMG parenchyma. US showed symmetric enlargement, inhomogeneous echogenicity, and dilated ducts of SMG (Fig. 1B). Mild lymphocytic infiltration between slightly dilated intralobular ducts was found on histologic evaluation (Fig. 1C). IgG4-positive cells were not found using immunohistochemistry (Fig. 1D). During the time period between the examination and tests, symptoms were relieved by 10 mg of prednisolone once a day for a week. We suspected this disease as eosinophilic sialodochitis. Thus, we prescribed 5 mg levocetirizine for two weeks. SMG pain and swelling subsided by antihistamine medication. After four more weeks of medication, sizes of both SMGs were nearly normal without aggravation. Blood examination revealed a normal eosinophil level (4.4%). His symptoms were well controlled by taking antihistamine medication intermittently for 7 months.
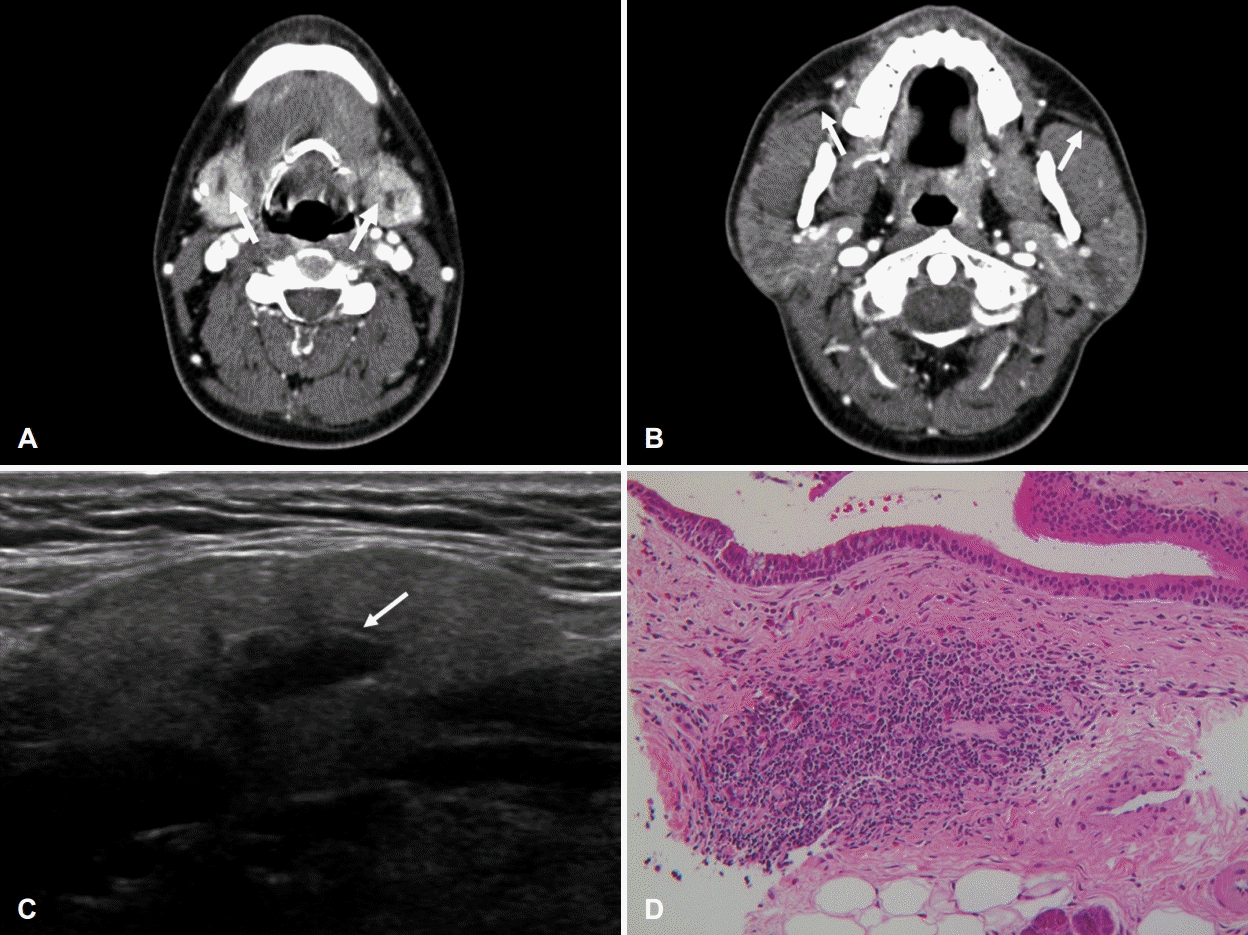
A 39-year-old male presented with symptoms of recurrent swelling of both SMG and parotid glands with pain for the past 10 years. When salivary glands swelled up, he complained of an itching sensation on the overlying skin of salivary glands. He reported no history of underlying disease including allergic disease. On physical examination, both SMGs and parotid glands were enlarged without tenderness. There was no palpable cervical node. CT revealed enhancement of both SMG parenchyma (Fig. 2A) and dilatation of both Wharton’s ducts. Dilation of both Stensen’s ducts was also observed on CT (Fig. 2B). Results of laboratory test showed elevated serum amylase (111 U/L, normal range: 28-100 U/L) and serum eosinophilia (5.3%, normal range: 0%-5%) without IgE elevation. Other laboratory tests for autoimmune diseases (anti-SS-A/Ro Ab, anti-SS-B/La Ab, RF, ANA, IgG, and IgG subclass IV) all showed negative finding. US followed by CNB were performed. US showed heterogenous echogenicity of both SMGs and dilatation of the intraglandular ducts (Fig. 2C). Histologic evaluation of SMG parenchyma revealed moderate lymphocytic infiltration with many eosinophils around a markedly dilated interlobular duct (eosinophil count up to 70/high-power field) (Fig. 2D). We prescribed 5 mg levocetirizine for two weeks under the suspicion of eosinophilic sialodochitis. Both SMG and parotid swelling as well as the itching sensation improved. Over a period of six months, his symptoms improved without recurrence.
Although organ-specific eosinophilic inflammation diseases such as eosinophilic granulomatosis, Kimura disease, IgG4-related disease, and hypereosinophilic syndrome have been extensively investigated [1], more studies are still needed to understand the pathophysiology and clinical findings of salivary gland diseases related to the eosinophil. Eosinophilic sialodochitis is the only disease that has similar characteristics to eosinophilic diseases of other organs in our literature review. Two patients of this report have specific features of eosinophilic sialodochitis.
Symptoms of eosinophilic sialodochitis are thought to be caused by saliva drainage failure due to mucus plugs, which contain rich eosinophils. The major mechanism of eosinophilrich mucus formation is understood as an allergic response to the refluxed allergen through the major salivary gland duct, which leads to eosinophilic ductal inflammation [5]. Therefore, symptoms related to allergic reactions are also frequently found in patients with eosinophilic sialodochitis. The incidence of allergic symptoms in patients with eosinophilic sialodochitis was 66% (39/59 patients) and itching of the overlying skin of the affected salivary gland was reported in 92% (36/37) of patients [6]. One patient also complained of allergic symptom (i.e., itching of the overlying skin) in the present report. Thus, taking a detailed history of allergic symptoms or disease might be helpful for diagnosing eosinophilic sialodochitis. On physical examination, the identification of mucus plugs containing rich eosinophils in patients with recurrent paroxysmal swelling of the major salivary glands has been suggested as a diagnostic criterion for eosinophilic sialodochitis [1]. However, we did not assess mucus plugs because we lacked an understanding of this disease during the diagnosis. Therefore, bimanual palpation for draining the saliva from the major salivary gland, which is simple clinical procedure, is recommended to patients who complain salivary gland swelling combined with allergic symptoms.
Elevated eosinophil and IgE in blood are considered as hallmarks of allergic disease [7]. Serum eosinophilia was observed in 71% of eosinophilic sialodochitis patients in a previous study [1]. This finding was also found in both patients of the present report. Although IgE elevation was found in 72% of patients in a previous study (72%, 23/32), only one patient had Ig elevation in this report. However, since both eosinophil and IgE showed significantly increased levels in the blood of patients with an allergic disease, eosinophil and IgE might be markers for differential diagnosis of recurrent salivary gland disease. Since eosinophil-rich saliva can cause functional obstruction of salivary gland duct, duct dilatation with an enhancement along with the duct, the hallmark of sialodochitis, can be determined by contrast imaging such as CT and MRI, even if calculi are absent. The evidence of ductal dilatation resulting from functional obstruction on any type of imaging of the salivary gland duct, such as sialography or US, can also be helpful to determine sialodochitis [3]. In both cases in this report, contrast CT confirmed that the duct was dilated in the absence of calculi. In addition, duct dilation was observed on US. Therefore, sialodochitis should be confirmed on imaging study for patients with suspected eosinophilic sialodochitis.
A previous study has summarized characteristics of eosinophilic sialodochitis and suggested the following diagnostic criteria for eosinophilic sialodochitis: 1) history of recurrent swelling of the major salivary glands, 2) eosinophil-rich mucus plugs drained from the salivary duct, 3) elevated IgE and proportion of eosinophils in serum, 4) history of allergic atopic disease, 5) ductal dilatation of the major salivary glands, 6) eosinophil and lymphocyte infiltration around ductal epithelial cells on histologic evaluation, and 7) negative for IgG4- related disease. When patients have characteristics 1 and 2 mentioned om the above criteria, sialodochitis can be diagnosed. In addition, this disease can be diagnosed in patients having periductal eosinophil- and lymphocyte-rich inflammation and fibrosis with associated reactive ductal epithelial cells observed without IgG4 deposition in the salivary gland parenchyma. In this study, case 2 had characteristics 1, 6, and 7. Therefore, the diagnosis of eosinophilic sialodochitis in case 2 was thought to be appropriate. Case 1 only had characteristics 1 and 7. Since we administered prednisolone to the patient before CNB, CNB may reveal only lymphocytic infiltration between the slightly dilated intralobular ducts without eosinophils. Since case 1 has improved his symptoms without recurrent salivary gland swelling by antihistamine medication for a considerable period, case 1 was also suspected to be eosinophilic sialodochitis. However, eosinophilic sialodochitis is a rare disease. Therefore, the differential diagnosis with other diseases including autoimmune sialadenitis, salivary gland disease caused by eosinophil, or physiological swelling of salivary gland should be considered. Identifying history of allergic disease or symptoms, ductal dilatation with sialodochitis on imaging study, and eosinophil and lymphocyte infiltration with a ductal dilatation on histologic evaluation should be considered primarily in diagnosing eosinophilic sialodochitis.
Massage of salivary gland and hydration are generally recommended to treat recurrent salivary gland swelling even in bacterial sialadenitis [8]. Antihistamine medication is generally not recommended because of its anticholinergic effect [9]. As antihistamines are proven to be effective in neutralizing eosinophil infiltration, this can be a feasible treatment option for allergic and eosinophilic diseases. In contrast to the management of bacterial sialadenitis, previous studies recommended antihistamines, leukotriene receptor antagonist (LTRA), or steroids without antibiotic as an initial treatment for eosinophilic sialodochitis [1,4]. Antihistamine medication was effective in both the patients in this study. LTRA was not prescribed due to its potential adverse effects on neuropsychiatric fuctions [10]. Therefore, antihistamine may be an initial choice for managing eosinophilic sialodochitis. Although eosinophilic sialodochitis will also expected to be responded to long-term steroids, antihistamine may be more appropriate for eosinophilic sialodochitis considering complications caused by longterm use of steroids, even with low-dose of steroids [11].
Other treatments for eosinophilic sialodochitis include relieving functional obstruction using saline, steroid, or antibiotic irrigation and mechanical dilation of the salivary duct gland (sialodochoplasty or bougienage) [1]. Further, there are several reports of the eosinophilic sialodochitis diagnosis after salivary gland resection. Although these treatments may effectively relieve salivary stasis in the eosinophilic sialodochitis patients, they are invasive or may require frequent clinic visits. Therefore, we suggest that these treatments are considered for patients whose symptoms are insufficiently relieved by medications.
In conclusion, although eosinophilic sialodochitis should not initially be considered as the cause of recurrent salivary gland inflammation because of its rarity, it is better to note that eosinophilic sialodochitis can cause recurrent salivary gland inflammation. Understanding the clinical, laboratory, and imaging characteristics of eosinophilic sialodochitis will aid the differential diagnosis of atypical cases of recurrent salivary gland diseases.
Notes
Author Contribution
Conceptualization: Bo Hae Kim. Data curation: Do Hyung Kim. Formal analysis: Kang Min Han. Investigation: Sang Eun Lee. Methodology: Bo Hae Kim. Resources: Kang Min Han. Supervision: Bo Hae Kim. Writing—original draft: Do Hyung Kim. Writing—review & editing: Bo Hae Kim, Kang Min Han.
REFERENCES
1. Baer AN, Okuhama A, Eisele DW, Tversky JR, Gniadek TJ. Eosinophilic sialodochitis: redefinition of ‘allergic parotitis’ and ‘sialodochitis fibrinosa’. Oral Dis. 2017; 23(7):840–8.
2. Kussmal I. [Recurrent salivary gland tumor due to chronic fibrotic inflammation of the Stensen’s ducts]. Berlin Klin Wschr. 1879; 15:209–2011.
3. Flores Robles BJ, Brea Álvarez B, Sanabria Sanchinel AA, Andrus RF, Espinosa Malpartida M, Ramos Giráldez C, et al. Sialodochitis fibrinosa (Kussmaul disease) report of 3 cases and literature review. Medicine (Baltimore). 2016; 95(42):e5132.
4. Hayashi K, Onda T, Ohata H, Takano N, Shibahara T. Case of suspected sialodochitis fibrinosa (Kussmaul’s disease). Bull Tokyo Dent Coll. 2016; 57(2):91–6.
5. Okuda M, Ogami Y, Unno T. Sialodochitis fibrinosa (Kussmaul). Sialodochitis fibrinosa (Kussmaul). Jibi to Rinsho. 1975; 21(4):635–9.
6. Carey B, O’Neill N, Brown J, Escudier M, Hullah E, Beneng K, et al. Eosinophilic sialodochitis: An emerging atopic condition. Oral Dis. 2022; 28(3):648–56.
7. Koh HS, Lee KS, Han DH, Rha YH, Choi SH. Relationship between serum total IgE, specific IgE, and peripheral blood eosinophil count according to specific allergic diseases. Allergy Asthma Respir Dis. 2013; 1(2):123–8.
8. Motamed M, Laugharne D, Bradley PJ. Management of chronic parotitis: A review. J Laryngol Otol. 2003; 117(7):521–6.
9. Thompson DF. Drug-induced parotitis. J Clin Pharm Ther. 1993; 18(4):255–8.
10. Gorton HC, Webb RT, Kapur N, Ashcroft DM. Non-psychotropic medication and risk of suicide or attempted suicide: A systematic review. BMJ Open. 2016; 6(1):e009074.
11. Waljee AK, Rogers MA, Lin P, Singal AG, Stein JD, Marks RM, et al. Short term use of oral corticosteroids and related harms among adults in the United States: Population based cohort study. BMJ. 2017; 357:j1415.
Fig. 1.
Image and histologic evaluations in case 1. A: Axial CT (contrast) image of the neck showing duct dilatation with a strong enhancement along both Wharton’s ducts (arrows). B: Ultrasonographic image showing symmetric enlargement with inhomogeneous echogenicity and ductal dilatation of hilum (arrows). Hematoxylin and eosin-stained sections. C: Mild lymphocytic infiltration between slightly dilated intralobular ducts (×400). D: IgG4 immunohistochemistry showing no IgG4-positive cells (×400). IgG4, immunoglobulin G4.
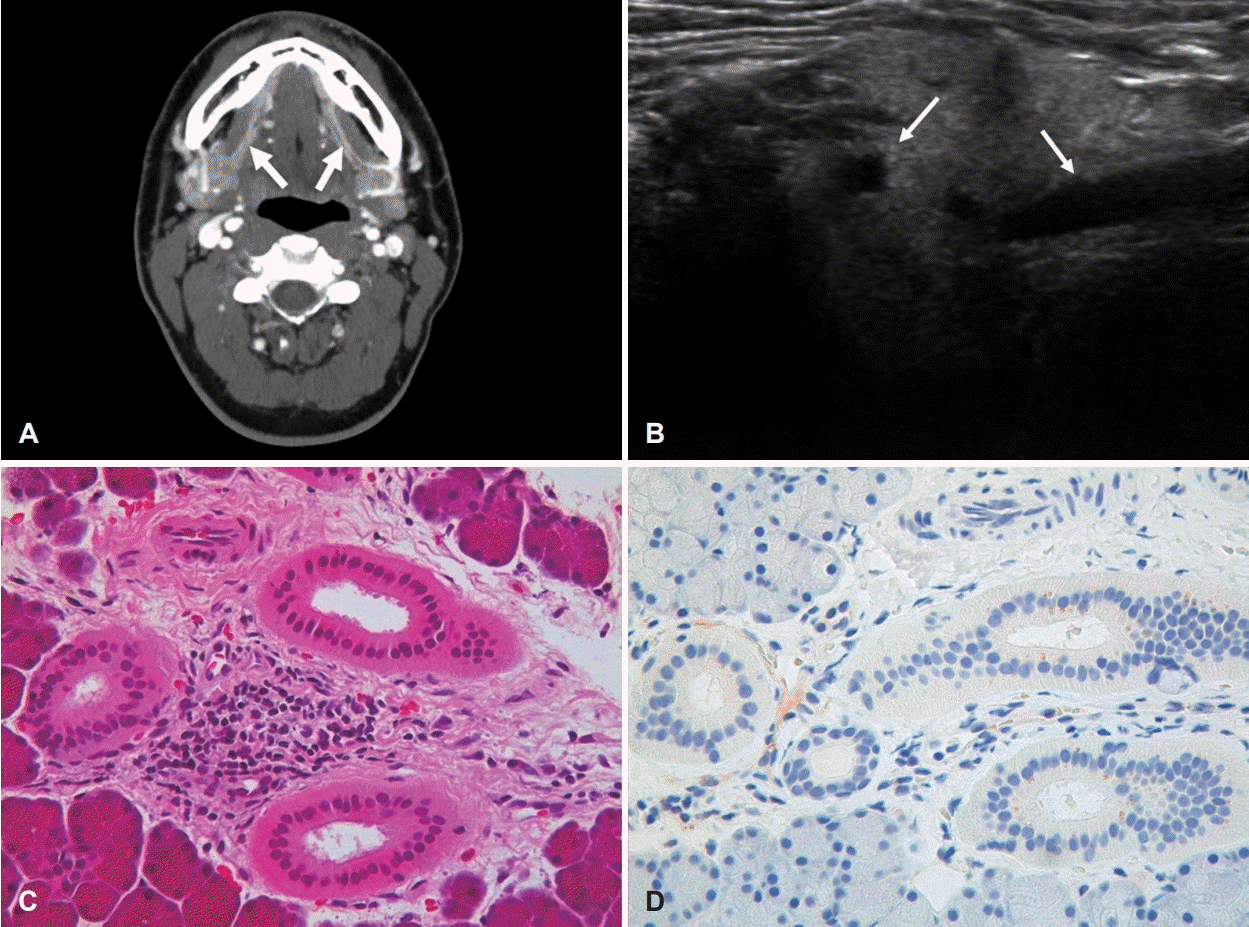
Fig. 2.
Image and histologic evaluations in case 2. A: Axial CT (contrast) image of the neck showing enhancement of SMG parenchyma and dilatation of both Wharton’s ducts (arrows). B: Axial CT (contrast) image of the neck showing dilation of both Stensen’s ducts (arrows). C: Ultrasonographic image showing heterogenous echogenicity of both SMGs and dilatation of intraglandular ducts (arrow). D: Hematoxylin and eosin-stained sections showing moderate lymphocytic infiltration with many eosinophils around a markedly dilated interlobular duct (×200) and eosinophil count up to 70/high-power field (×400). SMG, submandibular gland.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download