1. Holder JT, O’Connell B, Hedley-Williams A, Wanna G. Cochlear implantation for single-sided deafness and tinnitus suppression. Am J Otolaryngol. 2017; 38(2):226–9.
2. Kim DK, Bae SC, Park KH, Jun BC, Lee DH, Yeo SW, et al. Tinnitus in patients with profound hearing loss and the effect of cochlear implantation. Eur Arch Otorhinolaryngol. 2013; 270(6):1803–8.
3. Oh SJ, Goh EK, Choi SW, Lee S, Lee HM, Lee IW, et al. Audiologic, surgical and subjective outcomes of active transcutaneous bone conduction implant system (Bonebridge). Int J Audiol. 2019; 58(12):956–63.
4. Brkic FF, Riss D, Scheuba K, Arnoldner C, Gstöttner W, Baumgartner WD, et al. Medical, technical and audiological outcomes of hearing rehabilitation with the bonebridge transcutaneous bone-conduction implant: A single-center experience. J Clin Med. 2019; 8(10):1614.
5. Curca IA, Parsa V, Macpherson EA, Scollie S, Vansevenant K, Zimmerman K, et al. Audiological outcome measures with the BONEBRIDGE transcutaneous bone conduction hearing implant: Impact of noise, reverberation and signal processing features. Int J Audiol. 2020; 59(7):556–65.
6. Reinfeldt S, Håkansson B, Taghavi H, Fredén Jansson KJ, EegOlofsson M. The bone conduction implant: Clinical results of the first six patients. Int J Audiol. 2015; 54(6):408–16.
7. Zhao C, Yang J, Liu Y, Gao M, Chen P, Zheng J, et al. Horizontal sound localisation and speech perception in Bonebridge-implanted single-sided deafness patients. J Laryngol Otol. 2020; 134:814–21.
8. Lee HJ, Kahinga AA, Moon IS. Clinical effect of an active transcutaneous bone-conduction implant on tinnitus in patients with ipsilateral sensorineural hearing loss. Auris Nasus Larynx. 2021; 48(3):394–9.
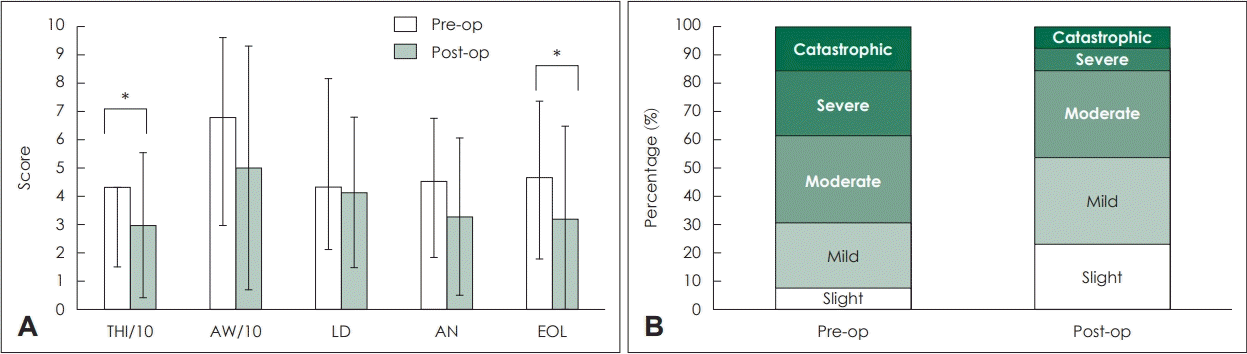
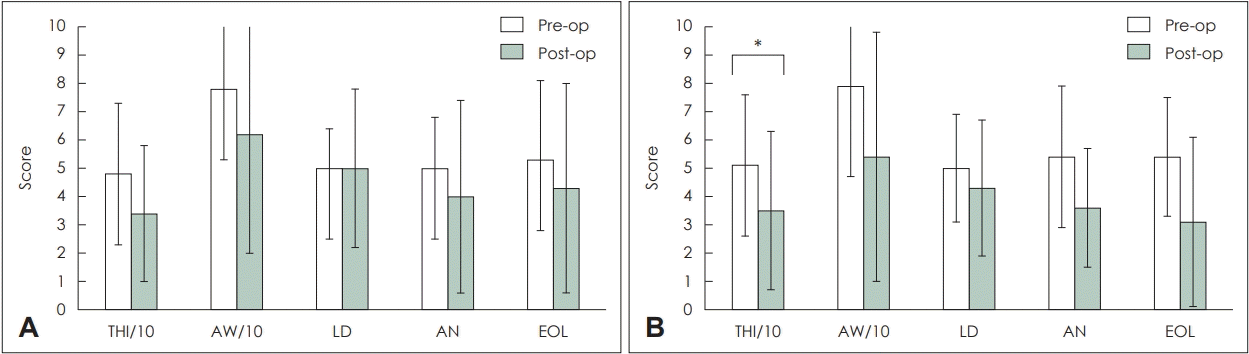
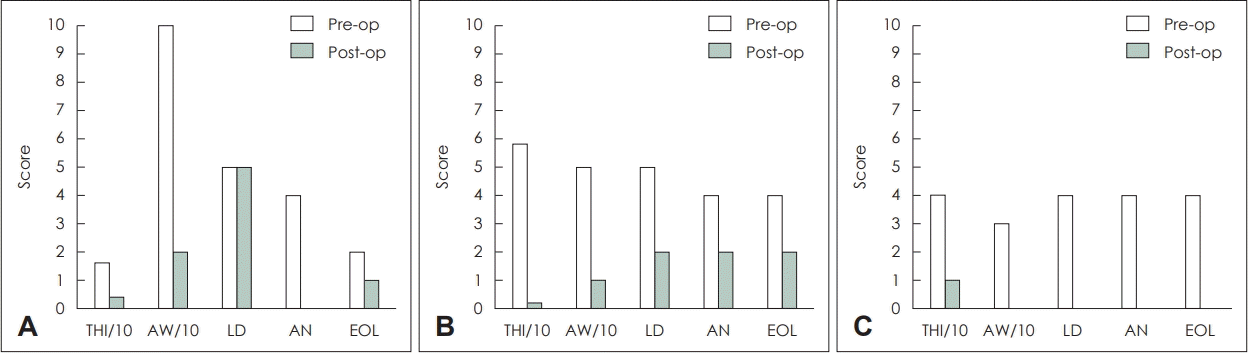
9. McCombe A, Baguley D, Coles R, McKenna L, McKinney C, Windle-Taylor P. Guidelines for the grading of tinnitus severity: The results of a working group commissioned by the British Association of Otolaryngologists, Head and Neck Surgeons, 1999. Clin Otolaryngol Allied Sci. 2001; 26(5):388–93.
10. Levy DA, Lee JA, Nguyen SA, McRackan TR, Meyer TA, Lambert PR. Cochlear implantation for treatment of tinnitus in single-sided deafness: A systematic review and meta-analysis. Otol Neurotol. 2020; 41(8):e1004–12.
11. Kloostra FJJ, Verbist J, Hofman R, Free RH, Arnold R, van Dijk P. A prospective study of the effect of cochlear implantation on tinnitus. Audiol Neurootol. 2018; 23(6):356–63.
12. Van de Heyning P, Vermeire K, Diebl M, Nopp P, Anderson I, De Ridder D. Incapacitating unilateral tinnitus in single-sided deafness treated by cochlear implantation. Ann Otol Rhinol Laryngol. 2008; 117(9):645–52.
13. Wang Q, Li JN, Lei GX, Chen DS, Wang WZ, Chen AT, et al. Interaction of tinnitus suppression and hearing ability after cochlear implantation. Acta Otolaryngol. 2017; 137(10):1077–82.
14. Bigelow RT, Reed NS, Brewster KK, Huang A, Rebok G, Rutherford BR, et al. Association of hearing loss with psychological distress and utilization of mental health services among adults in the United States. JAMA Netw Open. 2020; 3(7):e2010986.
15. Jastreboff PJ. 25 years of tinnitus retraining therapy. HNO. 2015; 63(4):307–11.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download